Key Points
- TBE often takes a severe clinical course in immuno-suppressed patients.
- In transplant patients TBE usually takes a fatal course.
- TBE vaccination in immuno-suppressed patients can be non-effective
- TBE in pregnancy has rarely been reported; from recent cases there is no evidence of transplacental infection of the offspring.
- The alimentary route of infection of TBE is still common in some European countries resulting in a high clinical manifestation index.
- TBEV can be infectious in milk and milk products for up to 14 days under optimal environmental conditions.
- TBE is an important travel-related disease. Increasing numbers of non-endemic countries report imported cases.
- Imported TBE cases in non-endemic areas pose challenges regarding the diagnosis of TBE.
TBE in immuno-suppressed patients
Changes in modern treatment regimens in hematology, oncology and autoimmune diseases significantly improved both quality of life as well as survival rates for numerous diseases. Modern approaches including hematopoietic stem cell transplantation (HSCT), solid organ transplantation, mono-clonal antibodies and target therapy are becoming more accessible, and thus the number of people living with immune-suppression continues to grow. It is well documented that there is a higher risk for this population for (severe) infections and this includes infections with the TBEV.
Currently, there are only few published cases of TBE in immunocompromised hosts, however, these show common patterns. Two fatal cases of patients treated with the anti-CD20 monoclonal antibody rituximab have been reported. The disease course in both cases was extremely fulminant with severe neurological symptoms and damage, and in both cases delayed antibody formation was observed.1 Two additional cases of rituximab-treated patients developing severe TBE were published by Steininger et al., indicating that TBE is a previously unrecognized severe infectious complication of rituximab therapy.2 In a recent case (Dobler, personal observation), a 22-year-old male patient suffering from non-Hodgkin lymphoma treated with rituximab developed clinically severe TBE one year after the end of a successful therapy. According to the treating physician the clinical course was life-threatening with fever and severe encephalitis. Finally, the patient survived with several months of convalescence and persistent neurological sequelae.
Expectedly, the inability to generate an antibody response renders rituximab-treated patients susceptible to TBE and it also impedes laboratory diagnosis. It is reported that in patients under rituximab therapy the antibody response is deficient for up to 6 months, making vaccination of these patients a challenge. The above case of a young patient who developed life-threatening TBE one year after rituximab medication was stopped shows that rituximab-induced B-cell response/antibody-deficiency may even last up to one year. Therefore, with patients receiving rituximab, information should be stressed on the importance of protecting themselves from tick bites and unpasteurized milk. There are no general recommendations to vaccinate patients against TBE before rituximab therapy. To obtain a good level of protection, repeated vaccine doses over time are needed, and this may not be possible in patients with an acute diagnosis of cancer. An accelerated schedule, with three doses on days 0, 7 and 21, has been used in some centers for patients with rheumatic diseases before initiating rituximab and can be recommended if the clinical situation permits.2 Still, the resulting protection rate remains unknown.
In a recent report an immunosuppressed patient in Italy had persistent viremia associated to the erythrocyte fraction of the blood as well as discharging the virus in the urine for more than six weeks, while receiving chemotherapy for relapsing blastic plasmacytoid dendritic cell neoplasm.3 Another dramatic case of TBE in a 12-year-old patient was published by Chmelik et al., where the immunosuppressive treatment regimen including dexamethasone and etoposide resulted in viral replication and fatal outcome.4 It has also been reported that thymectomized patients showed a delayed humoral immune response to TBE virus.5
Lipowski et al. recently described three patients who had received solid organ transplants from a single (undiagnosed viremic) donor (2 received a kidney, and 1 received a liver) and all organ recipients developed TBE-encephalitis 17–49 days after transplantation with fatal outcomes.6 The incubation period ranged from 17 to 51 days and thus was longer than what is seen in natural infections. The difference of 27 days between the two recipients of kidneys might result from the amount of virus in the respective donor organ. The presence of TBE virus was confirmed by real time PCR in all recipients and their donors, and direct sequencing of amplification products showed the presence of the same viral strain.
Table 1: Compilation of three patients with TBE after solid organ transplantation.
| Patient No. | Organ | Immuno-suppressive treatment | Incubation | Symptoms | Duration | Outcome |
|---|---|---|---|---|---|---|
| 1 | Liver | Steroids, tacrolimus | 17 days | Fever, meningitis, encephalitis, tetraplegia | 69 days | fatal |
| 2 | Kidney | Steroids, tacrolimus, mycophenolate mofetil | 22 days | Fever, meningitis, encephalitis, brain bleeding | 36 days | fatal |
| 3 | Kidney | Steroids, tacrolimus, mycophenolate mofetil | 49 days | Fever, meningitis, encephalitis | 83 days | fatal |
All three patients died. It remains unclear whether the differences in the clinical courses in the three patients were due to the non-natural transmission, the immune-suppression or both. Only one of the three patients showed the typical features of TBE in the cerebrospinal fluid, pleocytosis and increased protein concentration.
In another case (Dobler, personal observation), a 55-year-old male patient with a complete primary 3 dose vaccination against TBE followed by one booster dose several years before a liver transplantation, but was not boosted for 7 years, developed a fatal form of TBE presenting with fever, encephalitis and tetraplegia. No information on the incubation period or on the immunosuppressive therapy was available. The patient died after 5 days of mechanical ventilation with severe symptoms of encephalomyelitis. A tick in his garden, which adjoined a known natural TBEV focus, had infected the patient. This case gives evidence that the “natural route” of TBE-infection may result in severe and fatal disease in transplant recipients.
In a published study including 31 heart-transplant recipients, seroconversion rates and post-vaccination antibody titers were markedly reduced in comparison to the control group, and these findings served as evidence for recommending other protective measures against TBE virus infection (clothing, avoiding high-risk areas for travel) in these patients.7 This study also reported the safety of TBE-vaccination in the above-mentioned cohort of immune-suppressed patients.
In summary, based on clinical cases published, TBE appears to be more severe in immunocompromised patients with prolonged viral shedding and a higher risk for a fatal outcome, while standard vaccination and vaccination schedules appear to be less effective.
Vaccination against TBE for HSCT patients at risk, i.e. those living in or travelling to endemic areas, can be performed starting at 6–12 months after transplantation; however, due to the lack of data this cannot be recommended as a routine procedure.8 The assessment of the immunogenicity of TBE-vaccine in patients with rheumatoid arthritis treated with tumor necrosis factor-inhibitors (TNFi) and/or methotrexate (MTX) was recently carried out by Hertzell et al. In this study, individuals < 60 years of age were given three doses of vaccine at month 0, 1, 12; individuals > 60 years old received an additional priming dose at month 3, i.e. a total of four doses, while TBE neutralizing antibodies were assessed by a rapid fluorescent focus inhibition test. The results reveal an insufficient antibody response one month after a complete schedule of three or four doses, compared to healthy age- and gender-matched controls.9
In another study 29 HIV-infected patients were vaccinated against TBE. The vaccination schedule was modified by the inclusion of a fourth dose according to the schedule 0-1-2-9 months.35 The immune response depended on the CD4 counts of the vaccinees at the time of vaccination. With this schedule 85% of the vaccinated persons achieved protective antibody titers. The titers persisted at least for one year after the third vaccine dose.
In summary, based on a few published clinical cases, there are individual reports of patients with severe immuno-suppression (solid organ transplantation) who developed TBE via the infected organ or by tick bite. All known TBE cases in transplant patients showed a fatal course.
The incubation period in organ-transplant patients was longer than reported in tick-infected TBE patients with no underlying disease. The ICH does not show the typical findings of TBE-infection in cerebrospinal fluid. In one patient with immunosuppressive treatment for non-Hodgkin lymphoma about 1 year earlier, a life-threatening form of severe TBE was observed. It is, however, unclear whether the immuno-suppression was the reason for the severe form.
Therefore, in immunosuppressed patients with a risk of TBE infection the immunogenicity of TBE vaccination should be tested by neutralization test. Vaccinated ICH-patients who had received 3 primary TBE vaccine doses before immunosuppressive therapy was started and with continuing risk of TBE infection should be tested after the immuno-suppression was stopped and they should be re-vaccinated in case no neutralizing antibodies are detected.
TBE in pregnancy
Pregnancy is another situation with (physiological) immunosuppression (for review see Koutis et al., 201432) and it may also result in more severe forms of TBE. Although TBE is endemic throughout most of Europe and Asia with high incidence rates in some regions, there are only few data on TBE during pregnancy and its effect on the human offspring. The only available reference is one report on the occurrence of three TBE cases during pregnancy in the former German Democratic Republic.10 Three pregnant females developed TBE after drinking contaminated milk and developed a clinically overt TBE.
Two of the three cases described developed TBE during the early phase of pregnancy (week 8 to 10 of gestation). The third woman showed first clinical signs of TBE in week 24 of gestation. Diagnosis at that time was confirmed using a neutralization test; however, no detailed information on the diagnostic confirmation (e.g. fourfold titer increase etc.) is given. Two of the three females showed a severe form of TBE (myelitis, encephalomyelitis). One pregnant female showed only a febrile course of the disease. All three women survived without neurological sequelae. The outcome in the offspring of the two pregnant females with TBE early during pregnancy was unfortunate (see Table 2). No serological information is provided from any of the three neonates.
In a few more cases described in the Czech Republic and in Austria in the 1950s and early 1960s no specific serological TBE diagnosis could be made, but the diagnosis was made on the basis of clinical symptoms and the epidemiological situation.34 However, in all cases the newborns showed neither any signs of infection nor did they develop any clinically overt neurological acute or persistent symptoms.
In 2018 two additional cases of TBE during pregnancy were brought to the attention of the author of this chapter (manuscript in preparation). One woman was in week 20 of gestation when she developed a very severe form of TBE requiring mechanical ventilation for several days. She gave birth to a healthy child. Serological testing at the time of birth and 3 and 6 months later as well as virologic testing of mother and baby showed that antibodies of the mother were diaplacentally transferred to the baby and could be detected at birth; however, the infant never developed IgM as evidence of active infection and IgG antibodies significantly decreased during the first months of life.
In the case of a twin pregnancy in Sweden reported in 2018 (manuscript in preparation) the mother developed severe clinical TBE (encephalitis). Symptoms started in week 30 of gestation. The mother gave birth to twins at term. Both infants did not show any serological evidence or signs of active TBE infection.
In conclusion there are few reports on TBE in pregnancy. The two cases of TBE in the late second trimester from 2018 show that a diaplacental infection could not be detected.
This observation is confirmed by a case early in the 1960s where the infection in week 24 of gestation resulted in a healthy child with no evidence of TBE or any neurological symptoms.
There are two early reports from an outbreak in 1961 where two pregnant females were infected early during the 1st trimester. Both gave birth to children (one of them pre-term) with brain bleeding. The infection in these cases was via contaminated milk, however, which might modulate the clinical course of TBE. Furthermore, it is unclear whether the neurological symptoms resulted from TBE infection or from possible other causes.
Some other less well-documented TBE cases during pregnancy resulted in healthy neonates with no evidence of infection or neurological symptoms.
These cases also show that pregnancy may be associated with a more severe course of TBE, which may result from the immunological situation during pregnancy, where there is a kind of physiological immunosuppression.
Alimentary TBE
It has been known for a long time that TBE can be transmitted by contaminated milk. In fact, the first larger outbreak of TBE (in 1953) in Europe was milk-borne, described in Roznov, Czech Republic with more than 600 human cases.11 At that time TBEV-transmission by milk was more important than transmission by ticks and TBE was named, “Biphasic milk fever”. With increasing industrialization in milk production and dairy production the alimentary route of infection of TBE was more and more forgotten in industrialized European countries.
However, in the more agriculturally-based countries of eastern Europe this means of transmission was still present, although only a small proportion of patients became infected this way. During the last few decades there has been an increasing trend back to “natural production methods” of foods. With this tendency, milk-borne TBE outbreaks now are reported even from industrialized countries like Austria and Germany.12 However, there have been numerous milk-borne outbreaks in different European countries during the last decades.13 In Slovakia it is estimated that up to 20% of all TBE cases are transmitted by the alimentary route.14 In many of the milk-borne outbreaks it was reported that the manifestation index of clinical disease by the oral route was almost 100%. Experimentally, it was already shown in the 1960s that ruminants actively discharged TBEV in their milk when infected with the TBEV. There is a delay between the occurrence of viremia (first) and discharging TBEV to the milk (second).
Table 2: Summary of three TBE cases during pregnancy during a milk-borne outbreak of TBE in the former German Democratic Republic in 1961.
| Clinical course of mother | Outcome of mother | Age of gestation | Neutralization test | Outcome of newborn |
|---|---|---|---|---|
| fever | healthy | week 8 | positive | Pre-term (gestational week not provided) with intracranial bleeding |
| myelitis | healthy | week 10 | positive | Birth at week 40 of gestation with intracranial bleeding |
| meningoencephalomyelitis | healthy | week 24 | positive | Birth at week 40 of a healthy newborn |
In experimentally-infected goats the concentration of discharged TBEV is higher than the concentration of the virus during viremia in the animal, which implies an active replication and discharging TBE virus in the mammary glands.15 Goats discharge the highest amount of TBEV, followed by sheep and then cows.16,17 Nevertheless, single cases and small outbreaks caused by cow milk have been observed.18
So far, it is unclear whether the milk of a breast-feeding woman can also transmit the TBEV to the infant. There is one such case, where there is a high suspicion that milk of the mother might have infected the infant:19 the infant developed TBE on the 10th day of life. Two days later the mother also suffered from TBE. The infant had only been fed with the milk of the mother. Although it is possible in principle that the child had been infected transplacentally, the course of the disease more likely suggests a milk-transmitted infection with a short incubation period, while the mother had been infected by a tick bite. Therefore, breast-feeding females should be cautious when being exposed to ticks in TBE-endemic regions.
Figure 1: TBEV titers in milk and blood in a goat after subcutaneous experimental infection
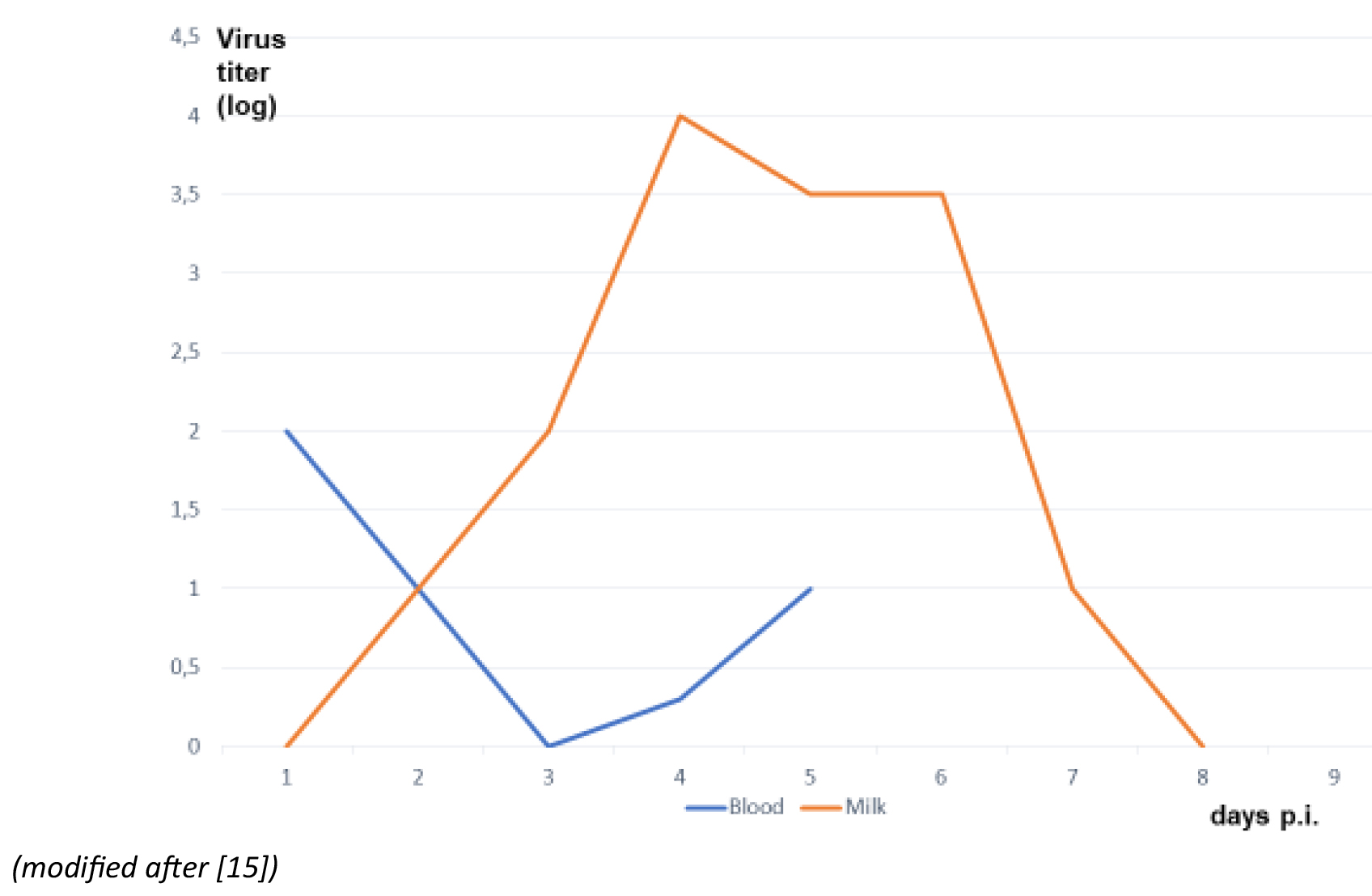
Click the image above to enlarge
There is evidence that TBEV is stable in milk and cheese for up to 14 days, depending on environmental conditions.20,21 At 4°C TBEV might remain viable up to 14 days, while at room temperature virus titers decrease after some days.20 There is evidence that the adsorption of TBEV in the human gut takes place in the duodenum,34 and moreover, TBEV seems to be protected by milk proteins during the stomach passage with its acid conditions, finally infecting duodenal cells.
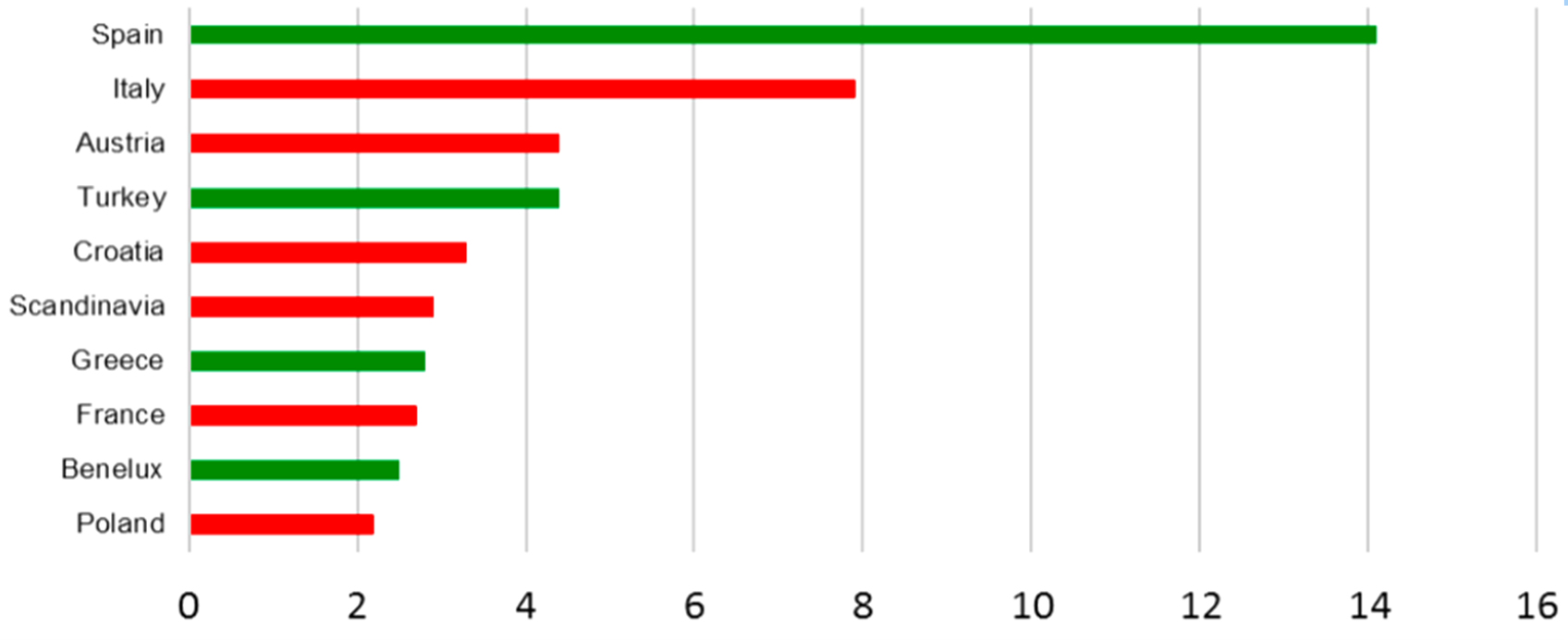
Figure 2: Travel targets/100 travelers in German travelers for 2017
Click the image above to enlarge
Click the image above to enlarge
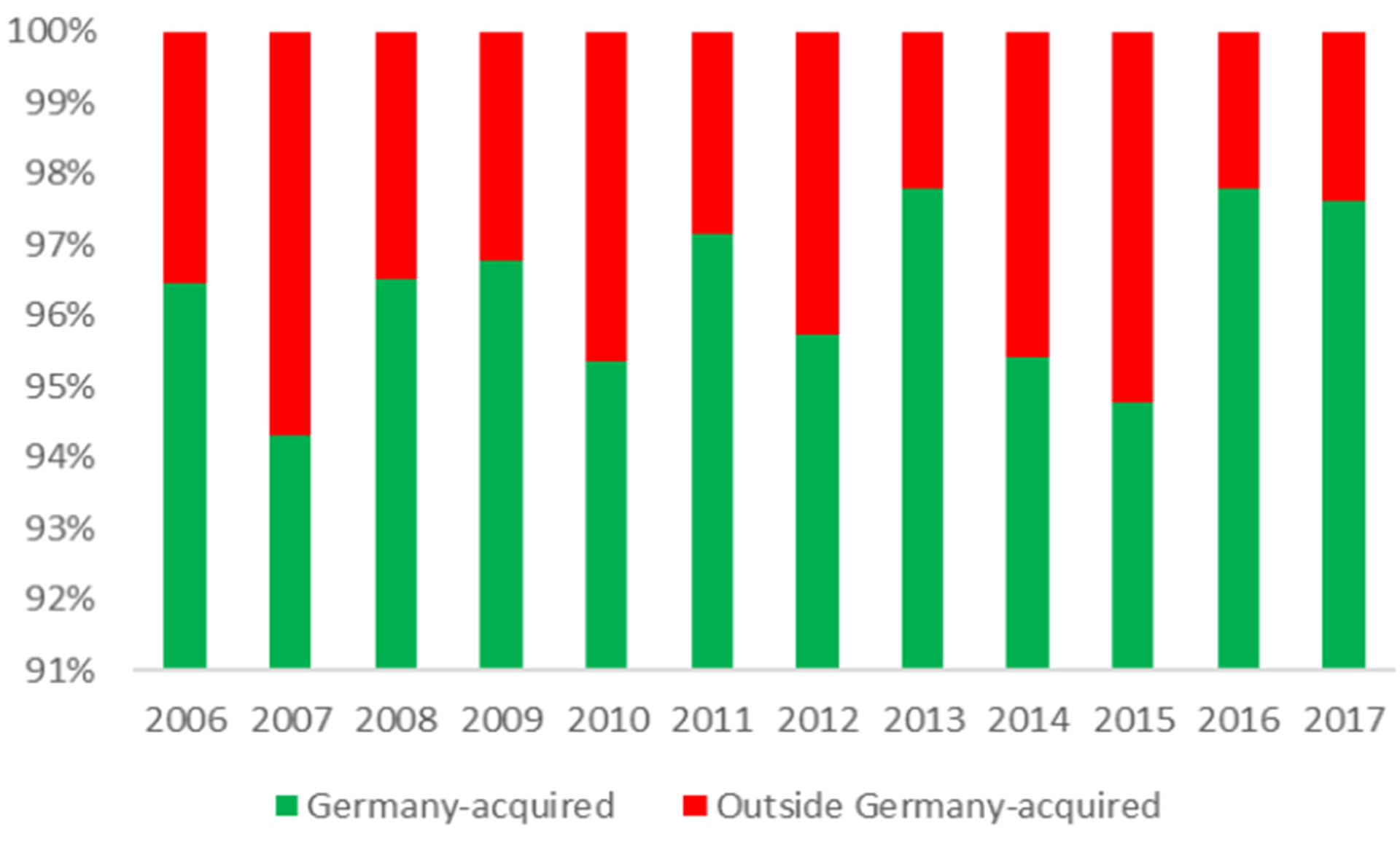
Figure 3: Percentage of travel-associated TBE cases in German patients
Click the image above to enlarge
TBE as travel risk
TBE is endemic in some of the most popular holiday destinations in Europe. In six of the 10 most visited countries TBE is endemic at least in some areas (Figure 2), including Austria and Scandinavia. In Germany about 2 to 6% of the annual TBE cases are acquired abroad (Robert Koch Institut Jahrbuch 2006 – 2017). Countries where Germans acquire TBE outside their own territory are ranked by frequency in Figure 4.
According to Süss22 the risk of infection with TBE after a tick bite in endemic areas varies according to the respective human activity from 1:200 to < 1:1000. Es in TBE endemic areas.23 In an Austrian study the risk of TBE infection was calculated at 1:10,000 for a week stay in Austria26. The risk depends on the time of travel (e.g. summer vs. winter), the duration of the travel and the risk activities during traveling.24 The real number of travel-associated TBE infections is underreported for many different reasons, most importantly lack of awareness and under-
Travel-related cases in non-endemic countries have been reported during recent years from Israel, the Netherlands, Australia, United States and England. The Australian patient traveled by car from Moscow to Novosibirsk with ample opportunities for exposure in nature although it was unclear whether he was infected by a tick bite or by the alimentary route. He developed a generalized infection with drowsiness, fatigue and lower limb myalgia.27 Two Germans from Baden-Württemberg, father and son, acquired their TBE infections during a travel rest by drinking goat milk and eating goat cheese in Zwiefalten, southwestern Germany.13 The infection of the son was diagnosed several days later when back again in London, UK, where he was employed. The infection of the father was only diagnosed in Germany after the diagnosis of the son was available. French physicians reported a number of TBE cases acquired outside of the country, mainly on the other side of the Rhine River in the Black Forest in Germany. Single travel-related TBE cases have also been reported from Austria, Russia, Czech Republic and Sweden.28 While recently the first autochthonous TBE cases were reported from the Netherlands,29 the greater proportion of TBE cases diagnosed in the Netherlands are still imported cases in travelers. TBE infections are imported to the Netherlands mainly from Germany and from Austria.30,31 In 2019 the first autochthonous cases of TBE in the United Kingdom were reported in travelers coming back from the UK to Germany.36
Figure 4: Origin of travel-imported TBE cases in German patients
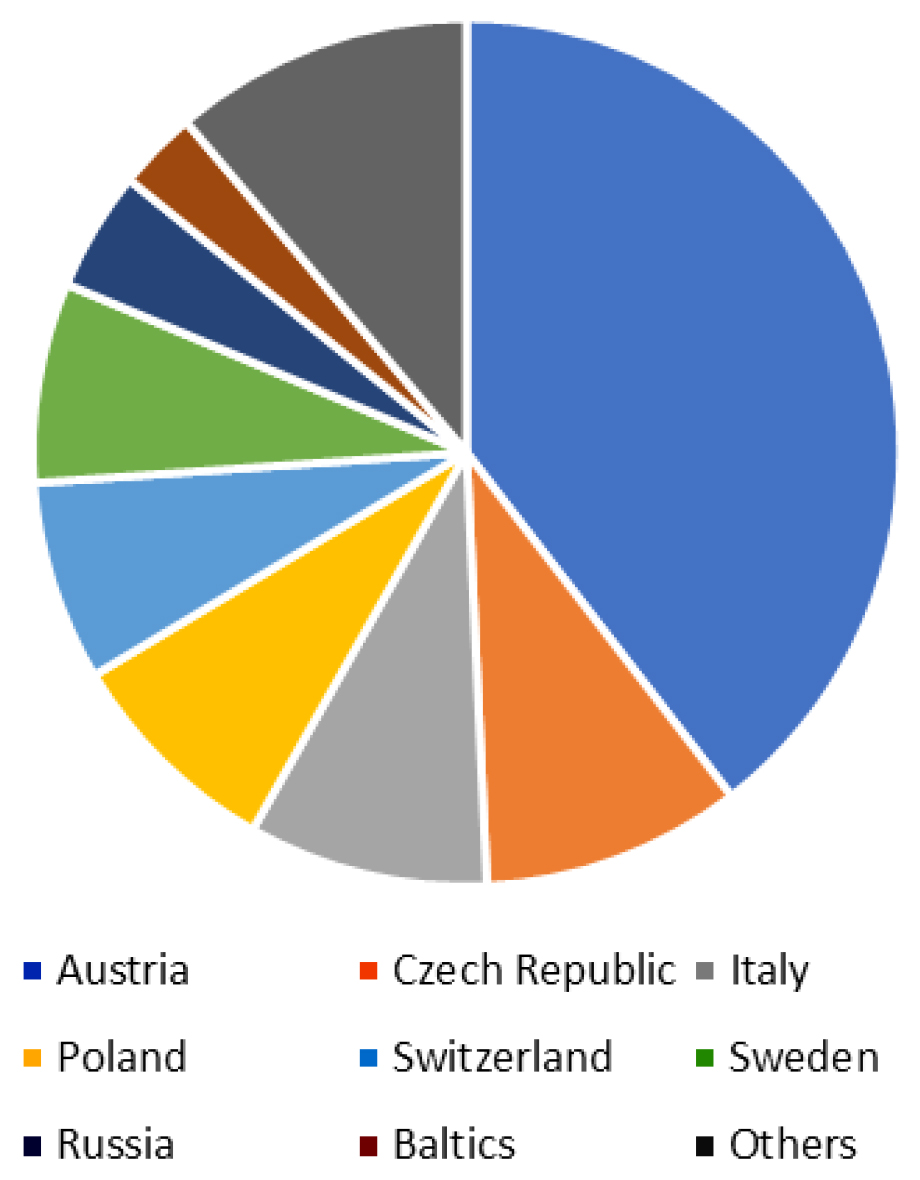
These examples show the importance of endemic holiday areas for the importation of TBE into non-endemic areas and the importance of the travel history in patients with encephalitis in order not to miss TBE in patients with CNS-infection.
Contact:
gerharddobler@bundeswehr.org
Citation:
Dobler G, Stoma I. TBE in special situations. Chapter 7. In: Dobler G, Erber W, Bröker M, Schmitt HJ, eds. The TBE Book. 6th ed. Singapore: Global Health Press; 2023. 10.33442/26613980_7-6
References
- Knight A, et al. Fatal outcome of tick-borne encephalitis in two patients with rheumatic disease treated with rituximab. Rheumatology. (Oxford). 2017;56(5):855-6. doi:10.1093/rheumatology/kew495
- Steininger PA, et al. Two Cases of Severe Tick-Borne Encephalitis in Rituximab-Treated Patients in Germany: Implications for Diagnosis and Prevention. Open Forum Infect Dis. 2017;4:(4):ofx204
- Caracciolo I, et al. Persistent viremia and urine shedding of tick-borne encephalitis virus in an infected immunosuppressed patient from a new epidemic cluster in North-Eastern Italy. J Clin Virol. 2015;69:48–51.
- Chmelík V, Chrdle A, Růžek D. Fatal tick-borne encephalitis in an immunosuppressed 12-year-old patient. J Clin Virol. 2016;74:73–74.
- Zlamy M, et al. Antibody dynamics after tick-borne encephalitis and measles–mumps–rubella vaccination in children post early thymectomy. Vaccine. 2010;28:8053–8060.
- Lipowski D, et al. A Cluster of Fatal Tick-borne Encephalitis Virus Infection in Organ Transplant Setting. J Infect Dis. 2015;215:896–901.
- Dengler TJ, et al. Vaccination against tick-borne encephalitis under therapeutic immunosuppression. Reduced efficacy in heart transplant recipients. Vaccine. 1999;17:7-8
- Hilgendorf I, et al. Vaccination of allogeneic haematopoietic stem cell transplant recipients: Report from the International Consensus Conference on Clinical Practice in chronic GVHD. Vaccine. 2011;29:2825–2833.
- Hertzell KB, et al. Tick-borne encephalitis (TBE) vaccine to medically immunosuppressed patients with rheumatoid arthritis: A prospective, open-label, multi-centre study. Vaccine. 2016;34(5):650–5.
- Helpert A, Sinnecker H. Ausgewählte Erhebungen zur Zeckenenzephalitis-Epidemie im Kreis Niesky, Bezirk Dresden, 1961. Deutsch Gesundheitswesen. 1966;21:1277-1279.
- Gresikova M, Rehacek J. Isolierung des Zeckenenzephalitisvirus aus Blut und Milch von Haustieren (Schaf und Kuh) nach Infektion durch Zecken der Gattung Ixodes ricinus L. Arch Ges Virusforsch. 1959;9:360-364.
- Holzmann H, Aberle SW, Stiasny K, Werner P, Mischak A, Zainer B. Tick-borne encephalitis from eating goat cheese in a mountain region in Austria. Emerg Infect Dis. 2009;15:1671-3.
- Brockmann SO, Oehme S, Buckenmaier T, Beer M, Jeffery-Smith A, Spannenkrebs M, Haag-Milz S, Wagner-Wiening C, Schlegel C, Fritz J, Zange S, Bestehorn M, Lindau A, Foggmann D, Tiberi S, Mackenstedt U, Dobler G. A cluster of two human cases of tick-borne encephalitis (TBE) transmitted by unpasteurised goat milk and cheese in German, May 2016. Euro Surveill. 2018;23(15):17-00336.
- Kerlik J, Avdicova M, Stefcovicova M, Tarkovska V, Pantikpva Valachova M, Molcanyi T, Mezencev R. Slovakia reports highest occurrence of alimentary tick-borne encephalitis in Europe: Analysis of tick-borne encephalitis outbreaks in Slovakia during 2007 to 2016. Travel Med Infect Dis. 2018;26:37-42.
- Gresikova M. [The transmission of the virus of the Czechoslovak tick encephalitis by goat milk]. In: Zeckenenzephalitis in Europa [Tick-borne encephalitis in Europe]. Libikova H, editor. Abhandlungen der Deutschen Akademie der Wissenschaften zu Berlin. Akademieverlag Berlin. Vol 1960;(2):121-2
- Gresikova M. Excretion of the tick-borne encephalitis virus in the milk of subcutaneously infected cows. Acta Virol. 1958a;2:188–192.
- Gresikova M. Recovery of tick-borne encephalitis virus from the blood and milk of subcutaneously infected sheep. Acta Virol. 1958b;2:113-119.
- Caini S, Szomor K, Ferenczi E, Szekelyne Gaspar A, Csohan A, Krisztalovics K. Tick-borne encephalitis transmitted by unpasteurised cow milk in western Hungary, September to October 2011. Euro Surveill. 2012;17:20128.
- Vaisviliene D. TBE in Lithuania. In: Süss J, Kahl O (ed.) Tick-borne encephalitis and Lyme borreliosis. 4th International Potsdam symposium on Tick-borne diseases. Pp. 100-113. Pabst Science Publishers. 1997.
- Offerdahl DK, Niall GC, Bloom ME. Stability of a tick-borne flavivirus in milk. Front Bioenerg Biotech 2016;4:40.
- Gresikova-Kohutova M. Effect of pH on infectivity of the tick-borne encephalitis virus. Acta Virol. 1959;3:159–167.
- Süss J. Epidemiology and ecology of TBE relevant to the production of effective vaccines. V 2003;21(suppl. 1):S19-S35.
- Steffen R. Epidemiology of tick-borne encephalitis (TBE) in international travellers to Western/Central Europe and conclusions on vaccination recommendations. J Travel Med. 2016;23(4). pii: taw018.
- Haditsch M, Kunze U. Tick-borne encephalitis: a disease neglected by travel medicine. Travel Med Infect Dis. 2013;11(5):295-300.
- Amato-Gauci AJ, Zeller H. Tick-borne encephalitis joins the diseases under surveillance in the European Union. Euro Surveill. 2013;17(42). pii: 20299.
- Rendi-Wagner P. Risk and prevention of tick-borne encephalitis in travellers. J Travel Med. 2004;11:307-312.
- Chaudhuri A, Ruzek D. First documented case of imported tick-borne encephalitis in Australia. Intern Med J. 2013;43(1):93-6.
- Hansmann Y, Velay A. TBE in France. In: Dobler G, Erber W, Schmitt HJ (eds.) Tick-borne encephalitis (TBE). 165-169. Global Health Press Pte Ltd, Singapore, 2018
- de Graaf JA, Reimerink JH, Voorn GP, Bij de Vaate EA, de Vries A, Rockx B, Schuitemaker A, Hira V. First human case of tick-borne encephalitis virus infection acquired in the Netherlands, July 2016. Euro Surveill. 2016;21(33).
- Reusken C, Reimerink JH, Verduin C, Sabbe L, Cleton N, Koopmans M. Case report: tick-borne encephalitis in two Dutch travellers returning from Austria, Netherlands July and August 2011. Euro Surveill. 2011;16(44). pii:20002.
- Hoornweg T, Reimerink JH. TBE in the Netherlands. in: Dobler G, Erber W, Schmitt HJ (eds.) Tick-borne encephalitis (TBE). pp. 209-212. Global Health Press Pte Ltd, Singapore, 2018.
- Kourtis AP, Read JS, Jamieson DJ. Pregnancy and infection. N Engl J Med. 2014;370:2211-18.
- Spiess H, Mumenthaler M, Burkhardt S, Keller H. Zentraleuropäische Enzephalitis (“Zeckenenzephalitis”) in der Schweiz. Schweiz Med Wochenschr. 1969;90:277-82.
- Libikova H. Zur Frage des Saisonvorkommens der ZE in Mitteleuropa. In: Zentraleuropäische Enzephalits in Europa. Berlin, Akademie-Verlag, 1961. p 69.
- Panasiuk B, Prokopowicz D, Panasiuk A. Immunological response in HIV-positive patients vaccinated against tick-borne encephalitis. Infection. 2003;31
- Kreusch TM, Holding M, Hewson R, Harder T, Medlock JM, Hansford KM, Dowall S, Semper A, Brooks T, Walsh A, Russell K, Wichmann O. A probable case of tick-borne encephalitis (TBE) acquired in England, July 2019. Euro Surveill. 2019 Nov;24(47).