With Lyme disease cases climbing steadily in Canada, the lack of an available vaccine has become a pressing concern among infectious disease specialists.
Getting bitten by a tick means risking a potential infection with Lyme disease. If left undiagnosed or untreated early, the bacteria can spread deep into different parts of the body and linger for years. This delayed diagnosis can lead to a roller-coaster of symptoms affecting the nervous system, joints, heart, and even skin, often appearing and disappearing unpredictably.
Despite the growing concern about Lyme disease, current preventive measures are limited to using tick-repellent spray, using antibiotics and performing tick checks after spending time outdoors.
The repercussions of Lyme disease can be profound. Last month, a 30-year-old Quebec woman opted for doctor-assisted death after years of battling the illness. Stéphanie Lavoie found herself bedridden in agonizing pain after contracting Lyme disease from a tick years ago. She said the intensity of her suffering led her to choose doctor-assisted death.
Despite the severity of Lyme disease in humans, the only available vaccine, LYMErix, was withdrawn from the U.S. and Canadian markets in 2002 due to low sales and what experts have called “negative publicity.”
“The LYMErix vaccine was authorized in 1998 by the United States Food and Drug Agency, and it was unfortunately recalled from the market by the drug manufacturer just three years later,” Raghu Venugopal, an emergency doctor in Toronto, told Global News.
“And really at the heart of the story of the Lyme vaccine saga is that medical doctors and also probably medical journalists did not do an adequate job in explaining to the public the benefits of the vaccine.”
There’s currently no Lyme disease vaccine available for humans. However, there are clinical trials taking place in Europe and the U.S., according to Health Canada.
“I think it’s really important that it be a national priority to have a vaccine,” Venugopal said. “Most dog owners know that there are readily available vaccines for dogs for Lyme disease. And it’s just a shame that there’s not one for humans.”
Lyme disease in Canada
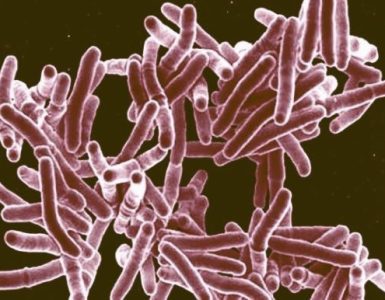
Lyme disease is caused by a corkscrew-shaped bacteria called Borrelia burgdorferi and is transmitted to humans through the bite of infected black-legged ticks. Symptoms include fever, headache, fatigue and a skin rash. If left untreated, the infection can spread to joints, the heart and the nervous system.
The disease has been around in Canada since the 1980s. But warmer winters over the last few decades have allowed ticks, and the pathogens they often carry, to flourish in ways they never did before, the Public Health Agency of Canada (PHAC) stated on its website.
The latest national Lyme disease data from 2023 shows 2,544 reported cases in Canada. However, according to PHAC, this preliminary count is likely an underestimation of the final total. A decade ago, there were only 632 reported cases in 2013.
Quebec coroner begins inquest into suicide of 22-year-old woman with Lyme disease
Lyme is traditionally associated with summer when ticks are most active. However, with warmer winters becoming the norm, the risk of contracting the disease extends beyond these months. Health Canada warns that ticks can be active whenever the temperature stays above freezing and the ground isn’t snow-covered, creating a year-round risk.
And tick exposure isn’t just limited to the deep woods, Venugopal warned.
“In emergency departments in Toronto and across Ontario this time of year, we see a lot of patients with tick exposures. So these are children, these are adults,” he said. “And it’s not quite that they were in the woods in grassy fields. I’ve seen patients with tick exposures who are walking on a path. I’ve seen people with tick exposures, like ticks on them, who have not been in the woods at all.”
LYMErix was a vaccine developed by GlaxoSmithKline (GSK) and approved for use in the United States and Canada in the late 1990s. It showed about 76 per cent efficacy in preventing Lyme disease after a complete series of three injections. However, despite its potential, GSK voluntarily pulled the vaccine from the market in 2002.
“There were certainly limitations with the vaccine. It wasn’t perfect, but no vaccine is,” infectious diseases specialist Dr. Isaac Bogoch said. “It was eventually pulled off the market, but there was not a lot of merit for this. It seemed like it just had a lot of negative publicity.”
When LYMErix was first rolled out in the U.S., the vaccine offered an effective prevention strategy for those at high risk for Lyme disease (living in endemic areas and spending a lot of time outdoors). However, around a year later, reports of adverse reactions occurring after vaccination started to circulate — including complaints of arthritis.
Driven by growing concerns over vaccine safety, a class-action lawsuit was filed against GSK in 1999. A Philadelphia-based law firm represented 121 individuals who claimed they experienced significant adverse reactions to the Lyme vaccine. According to the law firm, the plaintiffs’ primary objective was to remove the vaccine from the market.
GSK eventually settled the lawsuit. The final agreement included over $1 million in legal fees for the prosecuting lawyers, but provided no financial compensation to the plaintiffs.
“But the complaints of these small number of patients about their arthritis symptoms led to negative press, and led to a lot of people being afraid of the Lyme vaccine,” Venugopal said.
He added that 1.4 million people in the U.S. got the LYMErix vaccine, and within that number, 121 complained of arthritis pain.
The FDA took these complaints seriously, Venugopal explained, and it reviewed all the data. Its investigation revealed that the incidence of arthritis among those who received the Lyme vaccine was the same as in the baseline population of those who did not receive the vaccine.
No evidence was found that LYMErix was causing harm, but production ceased in 2002 due to a lack of demand.
“When patients have a benefit from the vaccine, it’s a non-medical event. It means that something doesn’t happen,” Venugopal said. “And that’s much more difficult to communicate to the public than someone having a vaccine side effect or illness occurring. And so what we see with the original Lyme vaccine developed in 1998 was that there really wasn’t a good explanation to the public how they were benefiting.“
Adding to its limitations, the LYMErix vaccine hadn’t been tested in children during initial trials. This restricted its approval to those aged 15 to 70 and living in high-risk Lyme disease areas.
“And children are a prime target for ticks, so it was not a children’s vaccine,” Venugopal said, adding this also continued to LYMErix’s downfall.
Why isn’t there a vaccine now?
The pharmaceutical company Pfizer and French biotech firm Valneva are working together to develop a new vaccine to protect both adults and kids as young as five from the most common Lyme strains.
The vaccine is currently in Phase 3 clinical trials in Europe and the U.S., and Bogoch said he is “cautiously optimistic” that it will come into the Canadian market.
“Lyme reins supreme in tick-borne illnesses. There is certainly a need for a vaccine,” he said.
In an email to Global News, a Pfizer spokesperson said because there are currently no approved vaccines for humans to prevent Lyme disease, the company hopes to “address a significant unmet need by bringing forward a vaccine that could prevent this debilitating disease.”
“The Phase 3 trials are ongoing, and it involves a randomized, observer-blind, placebo-controlled Phase 3 trial which has enrolled over 9,000 participants five years of age and older to receive VLA15 or a saline placebo,” the spokesperson said.
“As part of the primary series, participants will receive three doses of VLA15 within the first year after starting the series, and one booster dose approximately one year after completion of the primary immunization. The final dose of the primary series is administered shortly before the peak Lyme disease season for the region. Participants will then be followed for the occurrence of Lyme disease.”
The trial is being conducted at sites located in areas where Lyme disease is highly endemic across the U.S., Canada and Europe and has enrolled volunteers with a cleared past infection with Lyme disease.
Pending successful completion of the Phase 3 trials, Pfizer could potentially submit the vaccine for approval in several markets, including Canada, the spokesperson added.
Janet Sperling, president of the Canadian Lyme Disease Foundation, stated that while a vaccine would be a valuable tool in the fight against Lyme disease, it wouldn’t be a complete solution.
“So my concern is that the vaccine is being seen as a silver bullet, but we’re not ready for that,” she told Global News. It’s premature to be talking about a vaccine when we’re still arguing about how do we define Lyme disease. How do we treat it?”
She worries that focusing solely on a vaccine could narrow the criteria for diagnosing Lyme disease, which is highly complex with a wide range of symptoms. Canada is still working to fully understand and address this complexity, she added.
How to stay safe
There are numerous ways to prevent tick bites, Bogoch explained. One method is to wear long, layered clothing when outdoors, although he acknowledges that this may not be ideal in the summer heat.
“You can also apply insect repellents containing DEET,” he said.
After spending time outdoors, Bogoch added, thoroughly check your body for ticks, paying special attention to areas such as underarms, behind the ears, inside the belly button, behind the knees, between the legs, around the waist and in the hair.
If you find a tick — which has an oval-shaped body with eight legs — use clean, fine-point tweezers to slowly pull it straight out. Wash the bite area with soap and water or an alcohol-based sanitizer. Contact your doctor if you feel unwell.
Since Lyme disease can often be prevented if antibiotics are started within 24 hours of the bite, doctors routinely prescribe medication to people with tick bites before confirming the presence of bacteria.
Ticks can be tested, but results often take at least two days. If antibiotics aren’t started within three days of the tick bite, they are less likely to prevent infection, according to Health Canada. Though still treatable, the infection may require more intensive treatment, sometimes necessitating up to four weeks of intravenous antibiotics.
Source: Global News