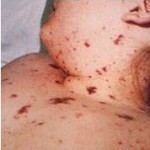
Positive progress has been announced in the development of a vaccine to eradicate deadly disease Meningitis B. The latest data supporting pharmaceutical company Novartis’ potential vaccine for the killer strain was revealed at the European Society for Paediatric Infectious Diseases (ESPID) in The Hague, Netherlands.
It shows that the vaccine, called 4CMenB, guarded against 80 percent of the 1,000 strains collected from across Europe. The study showed the vaccine worked safely alongside other vaccines when tested on 1,800 infants across Europe. Another study involving 1,500 toddlers showed the vaccine provides protection when used as a booster.
Meningitis B is the most common form of bacterial meningitis in the UK and is also one of the most deadly, in some cases killing in less than four hours.
The 4CMenB vaccine is awaiting authorisation from the European Medicines Agency (EMA) before it can be considered for introduction into the Routine Immunisation Programme in the UK.
Successful vaccines exist against some forms of meningitis, including Hib, Meningitis C and pneumococcal meningitis but, due to its complex nature, Meningitis B has been the hardest to immunise against and as yet there is no vaccine in use in the UK
Steve Dayman, Meningitis UK chief executive said: “This data is a very positive step in the fight to eradicate Meningitis B which is the biggest killer of all types of meningitis in the UK. It is anticipated that the EMA licence decision will be made around Christmas this year. There are still a number of questions to be answered about the vaccine and we are extremely hopeful that the decision will be positive. This vaccine is the first of its kind and has the potential to save thousands of lives.”
Dr Myron Christodoulides, Meningitis UK’s Scientific Medical Advisory Panel Chair and expert in microbiology and infection at the University of Southampton, said: “Novartis has taken us one step closer to the licensure of a vaccine with the potential to offer broad coverage against group B meningococcal disease.”
“Their candidate vaccine, 4CMenB, was developed using a state-of-the-art scientific approach to identify components of the meningococus that could be potential vaccine candidates. Indeed, new data from the immunogenicity studies in infants, toddlers and adolescents show that 4CMenB seems to have induced protective immune responses to meningococcal strains and equally importantly, the adverse effects to the vaccine were tolerable.”
“If this vaccine were to fulfil its potential to provide protection against 80 percent of Meningitis B strains causing disease in Europe, then it will significantly improve public health and save the lives of many from this devastating disease.”
Source: Meningitis UK

















