Another year, another flu vaccine because so far scientists haven’t managed to make a vaccine that protects against all strains of flu. A new approach could end that ritual and protect against deadly pandemic flu.
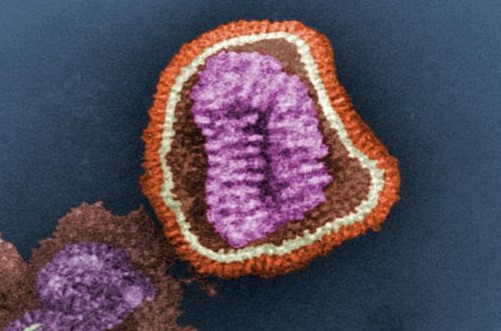
If the virus that causes flu were an ice cream cone, then the yearly vaccine teaches the immune system to recognize just the scoop – chocolate one year, strawberry the next. As the virus changes each year, so too must the vaccine.
A new approach that teaches the body to recognize the cone portion of the virus – which stays the same year-to-year – could shake up that yearly vaccination ritual and protect people against pandemic flu like the one that killed 40 to 50 million people in 1918. The team working on this new approach, led by Stanford biochemist Peter Kim, has shown early signs that their technique works in lab animals. They warn that they still need to make their vaccine more specific and show it works in much larger studies before testing it in people.
“We think it could be very generalizable,” said Kim, who is the Virginia and D.K. Ludwig professor of biochemistry and the lead investigator of the infectious disease initiative at the Chan Zuckerberg Biohub. “It could be important for coming up with a universal flu vaccine that would protect against pandemic flu, as well as for HIV.”
Focusing the immune system
First, a primer on flu vaccines. The idea is to inject a person with either a killed virus or just a single protein normally found on the virus surface. The immune system learns to recognize bits of that artificial invader, and mounts a defense that it can activate months or even years later if it sees that protein again. The challenge is that some portions of a protein are, for whatever reason, a lot easier for the immune system to detect. In the case of flu, that easily detected portion is the ice cream end, thus the annual vaccine against the flavor of the year. Try though they might, scientists haven’t been able to effectively direct the immune system’s attention to the cone.
The idea for the new approach came about when chemistry graduate student Payton Weidenbacher heard a talk about a protein that can bind very specifically to exactly the spot on the flu virus protein they want the immune system to recognize. (The protein is called a monoclonal antibody – “mono” because it binds to just one spot and “clonal” because scientists can make a lot of identical copies of it.) In the talk Weidenbacher attended, the scientists wondered if they could use the monoclonal antibody as a guide and create a way for the immune system to bind to the same spot.
Listening to the talk, Weidenbacher remembered a chemical trick that he thought might be a different approach. Instead of just learning from the monoclonal antibody, why not make use of it? His idea was to latch this highly specific monoclonal antibody onto the flu virus protein in the lab and use it as a stencil. He could paint the rest of the protein with molecules that act as a chemical cloak, rendering it invisible to the immune system. Removing the stencil would leave only a tiny portion of the protein visible for the immune system to learn to recognize and eventually attack.
Using that mostly cloaked protein as a vaccine may push the immune system to mount an attack against the cone – the portion of the virus shared across flu strains, including pandemic flu.
Start now
Weidenbacher mentioned his idea to Kim after the talk, but both assumed someone else would have thought of such a simple idea. Then, Weidenbacher got a late-night email from Kim. “Peter was like, ‘nobody’s done it, start now,’” said Weidenbacher, who joined Kim’s lab through a ChEM-H graduate program that trains students to apply chemistry know-how to problems in biology and medicine.
“Payton is a chemist,” Kim said. “What he did is come up with a way of using the monoclonal antibody not as something you look at but as a reagent.”
Although the idea was simple, carrying it out was not. Weidenbacher encountered some hurdles getting the system to work, but the team’s early tests, which they published April 26 in the Proceedings of the National Academy of Sciences, look promising. Lab animals that receive this cleverly cloaked flu protein also show an immune response to other strains of the flu – something that would only happen if they’d learned to recognize the consistent bits in the cone. Animals that received a normal vaccine didn’t respond well to other flu strains.
Kim and Weidenbacher said they’ve “skewed” the immune response, but they have work to do to get it to be more specific. But if they succeed, they said it could become an approach that works for many different infectious agents.
“You should be able to do this on anything – that’s the dream,” Weidenbacher said. “With the right chemistry, you could take any monoclonal antibody off the shelf and do this.”
Kim is also a member of Stanford ChEM-H, Stanford Bio-X, the Maternal & Child Health Research Institute and the Wu Tsai Neurosciences Institute.
This work was supported by the Virginia and D. K. Ludwig Fund for Cancer Research and the Chan Zuckerberg Biohub.
Source: Stanford News