Emerging infectious diseases are affecting public health and economies globally. Still living in the COVID-19 pandemic and now seeing another global public health emergency with monkeypox, the world must also be on full alert regarding Marburg Virus Disease (MVD) which is a highly contagious and deadly virus.
If a Marburg virus (MARV) outbreak goes out of hand, it might result in millions of deaths due to a case fatality rate of up to 90%. As with monkeypox, the disease is travelling from Africa to the rest of the world, and therefore there is an urgent need to screen travelers from endemic countries, to be aware of the clinical manifestations, and to accelerate the evaluation and approval of MVD vaccines and therapeutics.
MARV was first isolated in the city Marburg, Germany, in August 1967 when 31 cases of MVD were reported and caused an outbreak. It is believed that the MARV might have been introduced to Germany from Uganda by importation of infected African green monkey (Cercopithecus aethiops) tissue, needed for poliomyelitis vaccine development by the local vaccine manufacturer at the time.
MVD is mainly thought to be an animal-borne zoonotic disease. The Egyptian fruit bat is the most common viral reservoir and natural host of MARV. The virus can be transmitted from bats to humans through prolonged exposure in mines and caves inhabited by bat colonies. MARV can be transmitted animal-to-human or human-to-human through direct contact with blood, secretions, or bodily fluids of infected people or via surfaces contaminated with these fluids through broken skin or mucous membranes.
The disease can be divided into three distinct phases:
- The generalization phase,
- the early organ phase, and
- the late organ/convalescence phase.
The incubation period varies from 2 to 21 days, with an average duration of 5–9 days.
It begins abruptly with unspecific symptoms including fever, headache, vomiting, diarrhea, and severe myalgia. Muscle aches and pain are common features. Severe watery diarrhea, abdominal cramping and vomiting may be seen as early as the first week of illness. The look of patients at this phase has been described as showing “ghost-like” drawn features with deep-set eyes and expressionless faces, severe exhaustion, and lethargy.
The second week is often characterized by severe hemorrhagic manifestations such as hematemesis, hematochezia, and spontaneous bleeding from venipuncture sites. During this phase there is development of a non-pruritic erythematous rash that starts to be focal initially but later spread to different parts of the body.
The severe phase of the illness is marked by the presence of high fever. It is often accompanied with confusion, and irritability, leukopenia, lymphopenia, hypokalemia, thrombocytopenia, and elevated liver enzymes. With progression of the disease, the patient may experience kidney- and multiorgan failure and shock resulting in the case fatality rate (CFR) up to 90% as mentioned above.
Treatment and prevention
There is no approved vaccine or antiviral treatment for MVD. Supportive care includes balancing fluids and electrolytes, maintaining adequate oxygen levels and blood pressure, and replacing lost blood and clotting factors. MARVAC is a WHO-coordinated consortium for the development of MVD-vaccines. Several potential MARV vaccines are under investigations as well as the attempt to develop effective postexposure therapies.
Vaccine approaches for MARV include multidose, single-dose, fast-acting, live-attenuated nonreplicating, and replicating viral vector vaccine regimens. A recombinant vesicular stomatitis virus (VSV)-based vaccine expressing the MARV glycoprotein (VSV-MARV) rapidly protected hosts from MVD in animal models. Another vaccine candidate, MVA-BN-Filo, containing both Marburg and Ebola virus antigens was also reported to potentially protect against both hemorrhagic viruses.
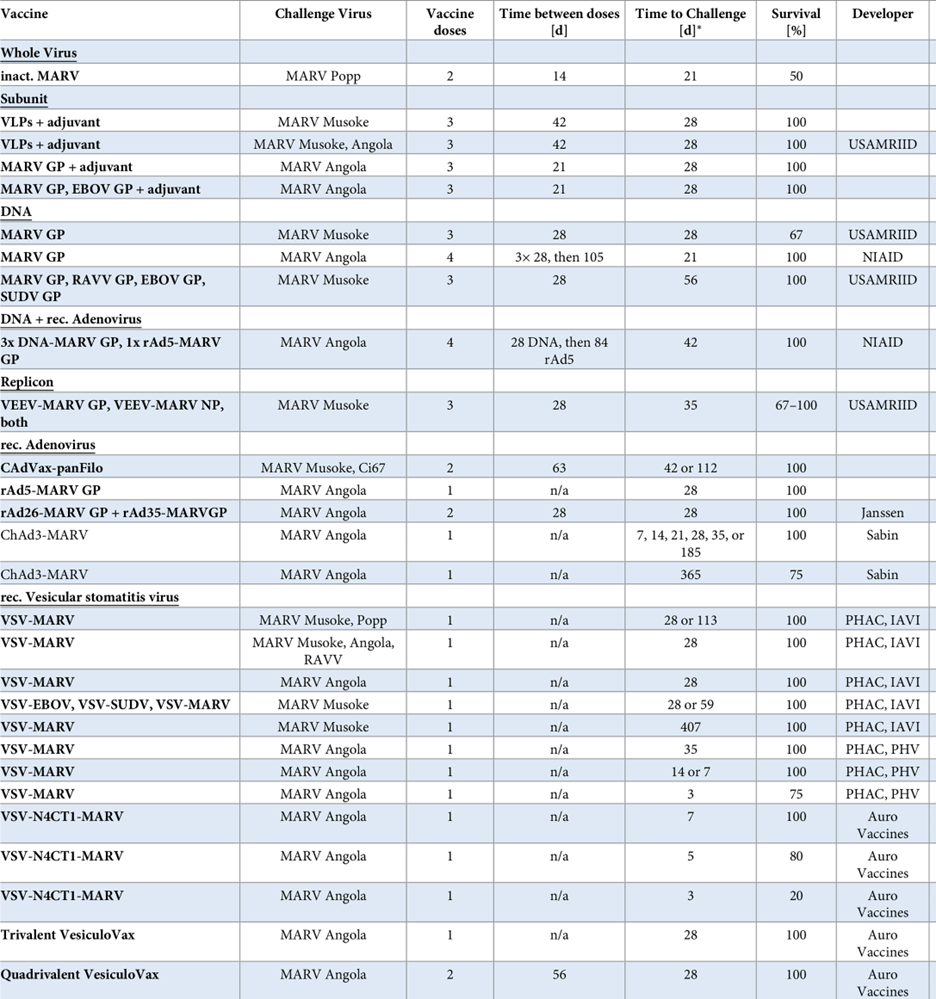
Table 1 MARV vaccines with protective efficacy in the preclinical NHP model
Click here for a complete list of references; Table taken from “An introduction to the Marburg virus vaccine consortium, MARVAC.” Published in PLOS Pathogens October 13, 2022
Besides prophylactic vaccines, postexposure therapies for MVD are under evaluation. Much of the research into MARV-specific postexposure treatments has centered upon the development of neutralizing and non-neutralizing antibody-based therapies targeting the MARV glycoprotein (GP). Additionally, small-interfering RNAs (siRNAs) targeting MARV messenger RNAs (mRNA) have been designed and evaluated in nonhuman primate (NHP) models. In addition to MARV-specific mAbs, broad spectrum small-molecule antivirals have been investigated for potential activity against MARV infection in vivo. Remdesivir (GS-5734) demonstrated an inhibitory activity in NHPs against several diverse lineages of RNA viruses, including members of the Filoviridae family (Marburg and Ebola Virus).
A combination therapy of the monoclonal antibody (MR 186-YTE) and an antiviral (Remdesivir) showed promising results in a non-human primate model. The development of MARV therapeutics is evolving at a much slower pace, but the following table shows some promising approaches with good postexposure efficacy in NHP’s.
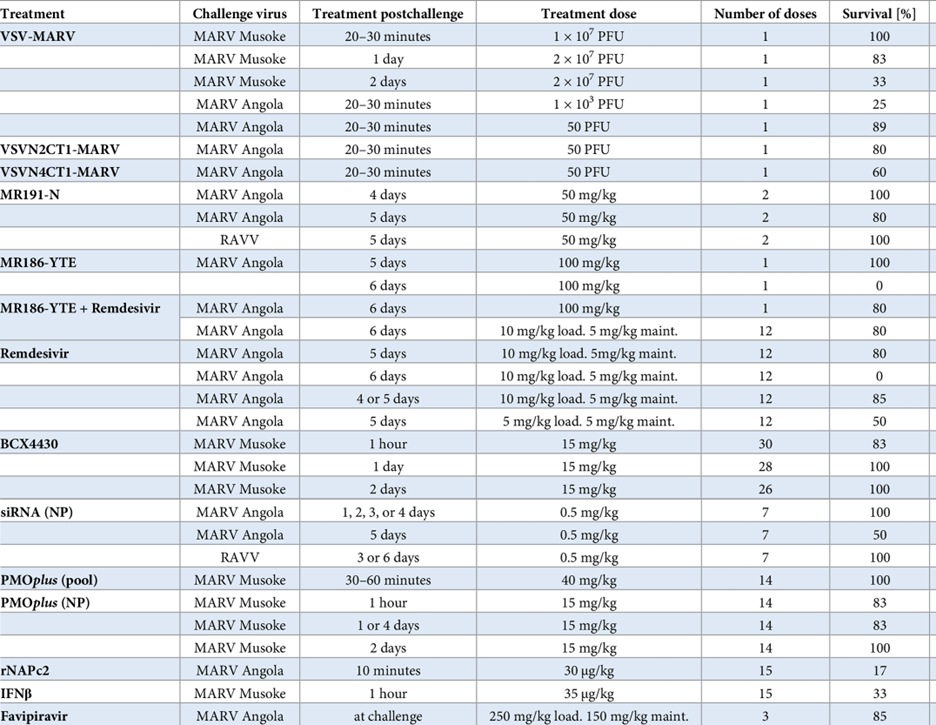
Table 2: MARV therapeutics with protective efficacy in the NHP model
Click here for a complete list of references; Table taken from “An introduction to the Marburg virus vaccine consortium, MARVAC.” Published in PLOS Pathogens October 13, 2022
Marburg Virus is a “risk group 4” pathogen due to its highly contagious nature and high CFR, and therefore there is an urgent need to develop effective solutions against this deadly virus. Having been a neglected infectious disease with smaller outbreaks over the past five decades, MARV may pose a high global public health concern if it spreads to non-endemic countries, and it may pose a high danger on the life of millions of people.
By Dr. Simone Müschenborn-Koglin Contributing Editor, GHP