Katrine M. Paulsen, Rose Vikse, Arnulf Soleng, Kristin S. Edgar, Heidi H. Lindstedt, Dagny H. Dorenberg, Berit Sofie Wiklund and Åshild K. Andreassen
E-CDC risk status: endemic
(data as of end 2022)
History and current situation
In Norway, tick-borne encephalitis (TBE) has been a mandatory notifiable disease since 1975 (Norwegian Surveillance system for communicable diseases, MSIS).1 According to ECDCs classification, coastal areas in southern Norway (counties of Agder, and Vestfold and Telemark) are endemic for TBE. Further, Viken County (former Østfold, Akershus and Buskerud), and western and northern Norway to Brønnøy municipality is imperiled.2-9
The first reported case of TBE occurred in 1997 at Tromøy in Agder County.10 This is a region with holiday cabins and outdoor recreation areas for both local inhabitants and tourists, and it is known for high temperatures during spring and summer. In addition, TBE antibodies in dogs and tick-borne encephalitis virus (TBEV) in ticks have been detected in this area.8,10-13
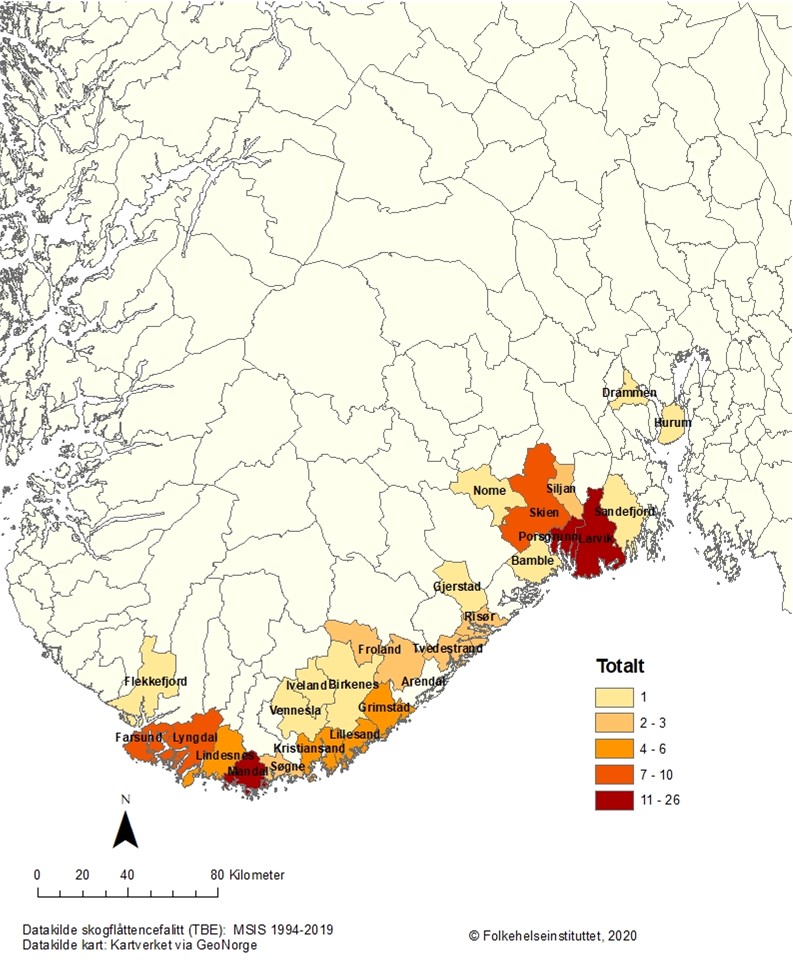
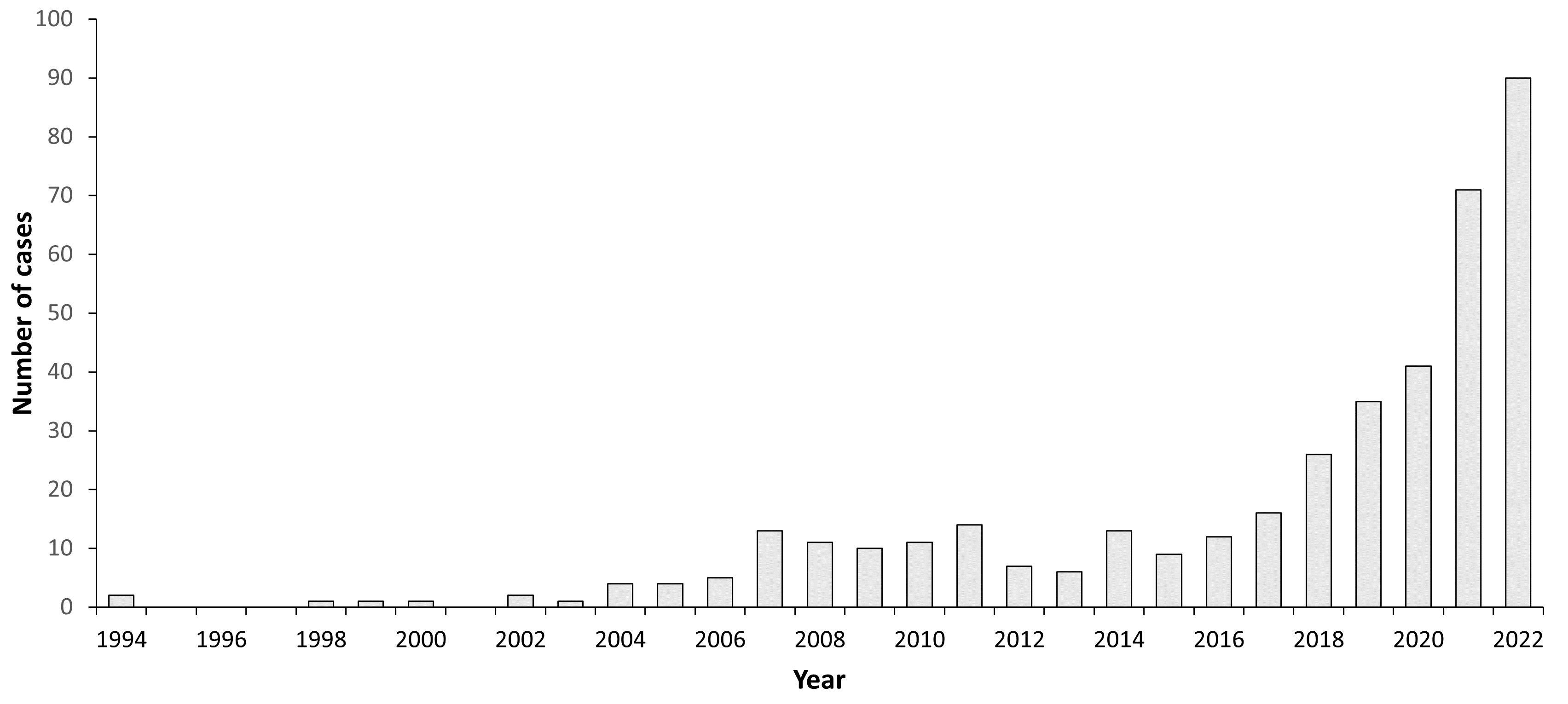
A total number of 245 TBE cases have been reported to MSIS per February 2021 (Fig. 1). Of these, 201 cases are autochthonous infections, while 44 cases were infected abroad or have an unknown infection history. The number of cases varies annually between 1 and 41 (Table 1 and Fig. 1). Data for 2018, 2019 and 2020 shows an increase in the number of cases, especially in the county of Vestfold and Telemark (MSIS, February 2021). The TBE patients’ age distribution is in accordance with other European studies, with a higher infection rate for those older than 30 years (Table 2 and Fig. 2).14-15 According to MSIS, the reported cases in Norway are represented by the counties of Agder, Vestfold and Telemark, and Viken, all located in the southern part of the country (Fig. 3). No cases are reported from the western or northern coastal areas, nor from the area east of the Oslofjord, even though outdoor recreation activities are common in the whole country.
Ticks and TBEV in Norway
The castor bean tick (Ixodes ricinus) is the most common tick species in Europe,16 and considered as the major vector of the European TBE-virus.17-18 The geographical distribution of I. ricinus in Norway has been examined in several studies.2,19-23 Both Tambs-Lyche (1943) and Mehl (1983) found I. ricinus to be mainly distributed in the coastal areas of Norway, from the southeastern border to Sweden, along the southern and western coastline, up to Nordland County at ~66°N.19-20 The density of ticks varies between locations, even when separated by short distances. This is probably caused by differences in microclimatic conditions, vegetation, and density of vertebrate hosts. However, locations with a high density of ticks are found all over the major distributional range. The density of ticks declines rapidly with both increasing distance from the coast and increasing altitude. In a multi-source study, Jore et al. (2011) suggested that tick populations in Norway had undergone recent shifts in latitudinal and altitudinal range.24 This result is, however, disputed in recent studies.2,21
Although ticks are reported far outside (i.e., northeast) of the hitherto established distribution limit of I. ricinus in Norway, the vast majority of these are engorged females.22-23 Migratory birds may deposit engorged larvae or nymphs in areas where temperatures permit development to the next stage but not completion of the life cycle. Thus, such records do not constitute evidence for established and sustainable tick populations as this requires the presence of all the active stages (larvae, nymphs, and adults) in a locality for at least two consecutive seasons.25-26 Using flagging and dragging, Soleng et al. (2018) found tick larvae, nymphs and adults to be abundant at 64.5 and 65.1°N. Only a few tick nymphs and adults, and no larvae, were found at locations close to 66°N. At several locations from 66.3°N up to 67.5°N no ticks were found.2 In a recent study by Hvidsten et al (2020), the occurrence of ticks in northern Norway was examined by dragging in 109 separate locations between the latitudes of 64°N and 70°N. The northernmost location with a permanent I. ricinus population was at 66.2°N on the Island of Dønna (Fig. 4).21 It is noteworthy that the taiga tick (Ixodes persulcatus) and the meadow tick (Dermacentor reticulatus) were not detected in a large screening of ticks collected in the southern part of Norway in 2016.27
Studies in I. ricinus in Norway have detected TBEV in nymphs with prevalence ranging from 0% to 1.1%. In adult ticks collected from the same areas, the prevalence ranges from 0% to 20.6%. TBEV positive ticks have been found in sampling areas along the Norwegian coastline from the east of Viken county to Brønnøy in Nordland county.6 The highest estimated TBEV prevalence in adult ticks has been found in the counties of Rogaland and Vestfold and Telemark. In nymphs, the highest prevalence has been found in Vestfold and Telemark, Agder and Rogaland.6
Historically, the first suggested TBEV isolate from Norway was collected in I. ricinus from Vestland County (former Sogn and Fjordane) in June 1976 as described by Traavik and coworkers. Five virus strains with close serological relationship to the TBEV complex were detected in this study.28
One pool of ten nymphs collected from southern Norway has been whole genome sequenced and phylogenetically characterized. The strain, “Mandal 2009”, was found to belong to the Scandinavian group of the European TBEV subtype. Interestingly, “Mandal 2009” revealed a shorter form of the TBEV genome within the 3’ non-coding region, similar to the highly virulent “Hypr” strain.29
Seroprevalence in animals
In addition to tick studies, a seroprevalence study has detected TBE antibodies in specimens from cervids (deer) collected in Farsund (Agder County) and Molde (Møre and Romsdal County). In Farsund, located on the southern coast of Norway, 41% (22 of 54 animals) were TBE-positive. This is in contrast to Molde, situated midwest, where the prevalence was 1.6% (1 of 64 animals). The same study detected antibodies to Louping ill virus (LIV), a closely related flavivirus, in 14.8% (8 of 54) of the analyzed cervid sera from Farsund.30
A recent seroprevalence study of cervids where serum samples were collected across Norway found TBEV antibodies in the municipalities of Steinkjer, Vindafjord, Søgne, Birkenes, Lardal, Larvik and Halden (Fig. 4). The overall seroprevalence was 4.6%. Antibodies against TBEV detected by serum neutralization test were present in 9.4% of the moose samples, 1.4% in red deer, 0.7% in roe deer, and 0% in reindeer.4
Ticks (6850 nymphs and 765 adults) from eastern, western, and northern Norway were analyzed for LIV using an in-house real-time polymerase chain reaction (PCR), none of these were positive (unpublished data). However, a recent study by Ytrehus et al. detected antibodies against LIV in willow ptarmigan (Lagopus lagopus lagopus) across the whole country. The study suggested that either LIV or a cross-reacting virus infects ptarmigan in Norway, also at high altitudes and latitudes.31
There is limited knowledge on TBEV in domestic animals in Norway. A recent study reported TBEV RNA in unpasteurized cow milk from three farms located in southern and northern Norway in 5.4% of the tested animals. Seropositive animals were only detected at one farm in southern Norway, in 88.2% of the tested animals.5 This is higher than in a previous study by Traavik (1973), where a seroprevalence of 17.7% was detected in bovine sera in western Norway.32
Seroprevalence in humans
Recently, a seroprevalence study in a TBE endemic area in southern Norway a TBEV seroprevalence of 3.1% (45/1,453) was found in the general adult population in Søgne municipality. Among individuals not vaccinated against TBEV and/or yellow fever, the seroprevalence of IgG antibodies to TBEV was 1.4% (6/419).33 Furthermore, a recent blood donor study from TBE endemic areas in Vestfold and Telemark found a low seroprevalence of 0.4% (4/1,123). Out of the 1,123 analyzed samples, 21 had neutralizing antibodies to TBEV, of which 17 reported a previous TBE vaccination.34
Three seroprevalence studies in humans from presumed non-endemic areas have been published. Larsen et al. detected TBE immunoglobulin G (IgG) antibodies among 0.65% of blood donors in Viken County (former Østfold) in southeastern Norway.9 The second study in 1,213 blood donors was performed in Vestland County (former Sogn and Fjordane), located in western Norway. TBE IgG antibodies (ELISA) were detected in five (0.4%) of these samples. However, four of these were reported to be vaccinated against flaviviruses and one was negative by neutralization test.35 In 1979, Traavik detected a 19.6% seroprevalence from Vestland County. However, these results were not confirmed with a neutralization test, and thus may be explained by cross-reactions to LIV, vaccine-related flaviviruses, or nonspecific binding in the test.36
Conclusion
In summary, TBE is endemic in Norway and the number of human TBE cases has been increasing in recent years. Clinical TBE cases are only found in southern parts of Norway; however, the results from both prevalence studies in ticks and seroprevalence studies in humans and animals indicate that TBEV might be widespread in the country, and not limited to the southern region. This is highly relevant information for public health considerations and risk evaluation. Further studies on tick distribution and prevalence of TBEV in ticks, humans and animals in Norway are currently ongoing.
Overview of TBE in Norway
| Table 1: Virus, vector, transmission of TBE in Norway | |
|---|---|
| Viral subtypes, distribution2-3,5-11 | Western subtype.
TBEV is distributed in Ixodes ricinus ticks in the following counties: Viken, Vestfold and Telemark, Agder, Rogaland, Vestland, Møre and Romsdal, Trøndelag, and Nordland. Human TBE cases have been reported in the following counties: Agder, Vestfold and Telemark, and Viken (three municipalities: Drammen, Vestby and Fredrikstad). [www.fhi.no Norwegian Surveillance System for Communicable Diseases (MSIS)] |
| Reservoir animals | Small rodents in the genera Apodemus and Myodes37 |
| Infected tick species (%) | I. ricinus (0%–1.1% in nymphs and 0%–20.6% in adults)6 |
| Dairy product transmission | Not documented. TBEV RNA has been detected in unpasteurized cow milk.5 |
Figure 1: Burden of TBE in Norway over time*
| Year | Number of cases | Incidence / 105 |
|---|---|---|
| 1994 | 2 | <0.1 |
| 1995 | 0 | 0 |
| 1996 | 0 | 0 |
| 1997 | 0 | 0 |
| 1998 | 1 | <0.1 |
| 1999 | 1 | <0.1 |
| 2000 | 1 | <0.1 |
| 2001 | 0 | 0 |
| 2002 | 2 | <0.1 |
| 2003 | 1 | <0.1 |
| 2004 | 4 | <0.1 |
| 2005 | 4 | <0.1 |
| 2006 | 5 | 0.1 |
| 2007 | 13 | 0.2 |
| 2008 | 11 | 0.2 |
| 2009 | 10 | 0.2 |
| 2010 | 11 | 0.2 |
| 2011 | 14 | 0.3 |
| 2012 | 7 | 0.1 |
| 2013 | 6 | 0.1 |
| 2014 | 13 | 0.2 |
| 2015 | 9 | 0.2 |
| 2016 | 12 | 0.2 |
| 2017 | 16 | 0.3 |
| 2018 | 26 | 0.5 |
| 2019 | 35 | 0.7 |
| 2020 | 41 | 0.8 |
| 2021 | 71 | 1.3 |
| 2022 | 90 | 1.6 |
| Table 2: TBE-reporting and vaccine prevention in Norway | |
|---|---|
| Mandatory TBE reporting | Hospitals and General Practitioners Only cases with meningitis/encephalitis are notifiable. Criteria:
and/or
Source: www.fhi.no |
| Other TBE-surveillance | Ongoing studies: The Barents region project (ID B1710).Emerging infections. Capacity building on vector-borne infections in the Barents Region, Norway and Russia.
EMERGING VIRUS: Vector-borne infections in Norway – Understanding the emergence of viral vector-borne diseases in a One Health perspective by studies of dynamics, distribution, climate, genetic diversity, biogeographic distribution, risk of infection, surveillance and diagnosis. Observation and prognosis of TBE-patients (Telemark Sykehus HF). North-Tick (Interreg VB, North Sea program): Vector-borne pathogens/infections in the North Sea area. Experimental infection study of sheep with TBEV: Transmission to lambs via milk38 TBFV net (EEA-project): surveillance and research on tick-borne flaviviruses Development of pipeline of whole genome sequencing of TBEV39 |
| Special clinical features | No. TBE has been mandatory notifiable to MSIS (Norwegian Surveillance System for Communicable Diseases) since 1975. Source: www.fhi.no |
| Available vaccines | TicoVac, Pfizer TicoVac Junior, Pfizer Source: The Norwegian Medicines Agency |
| Vaccination recommendations and reimbursement | TBE vaccination should be considered for children and adults who often experience tick bites in coastal areas where human TBE cases have been reported: – Sørlandet and the west coast of Oslofjorden from Flekkefjord to Drammen – The east coast of Oslofjorden from Vestby to the Swedish border Source: www.fhi.no |
| Vaccine uptake by age group/ risk group/ general population | In Norway, all immunizations should be registered into the national immunization register, SYSVAK. According to SYSVAK, 77,677 persons have received at least 3 doses of TBE vaccine. There is no information about risk factors in the register. For vaccines outside the childhood immunization program, registration into SYSVAK was consensual up to 1/1/2020. The number of TBE vaccine doses actually given could therefore be higher than the numbers registered. Source: Norwegian Immunisation Registry (SYSVAK) and Norwegian Institute of Public Health’s Warehouse Management System |
| Name, address/website of TBE National Reference Center | Norwegian Institute of Public Health Source: www.fhi.no |
Figure 2: Age and gender distribution of TBE in Norway 1994–2022*
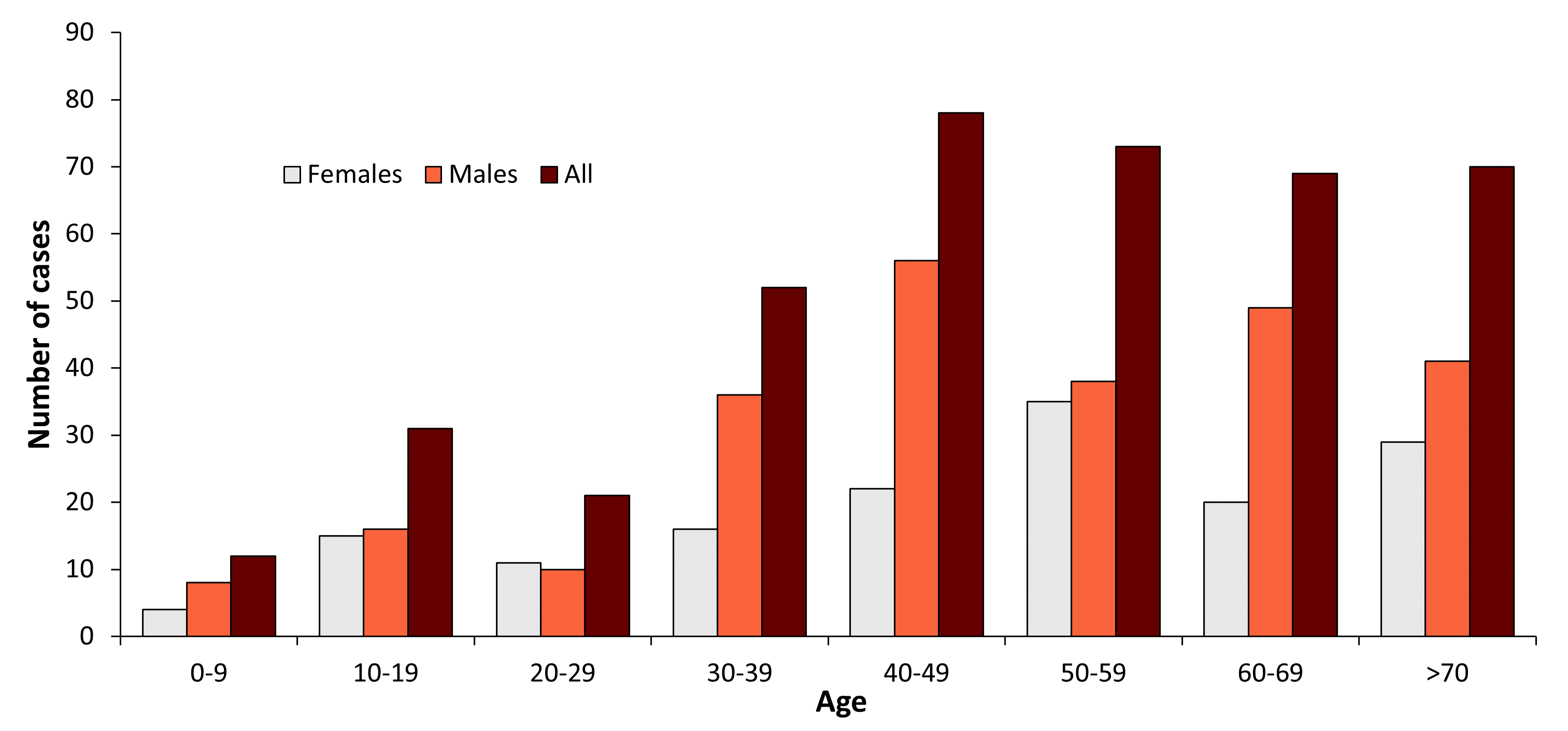
| Age groups (years) | Females | Males | All |
|---|---|---|---|
| 0-9 | 4 | 8 | 12 |
| 10-19 | 15 | 16 | 31 |
| 20-29 | 11 | 10 | 21 |
| 30-39 | 16 | 36 | 52 |
| 40-49 | 22 | 56 | 78 |
| 50-59 | 35 | 38 | 73 |
| 60-69 | 20 | 49 | 69 |
| 70+ | 29 | 41 | 70 |
These data include 46 cases that have been infected abroad or have an unknown infection history.
Figure 4: Geographical locations where tick-borne encephalitis virus has been detected in Norway from 2004 to 2020.
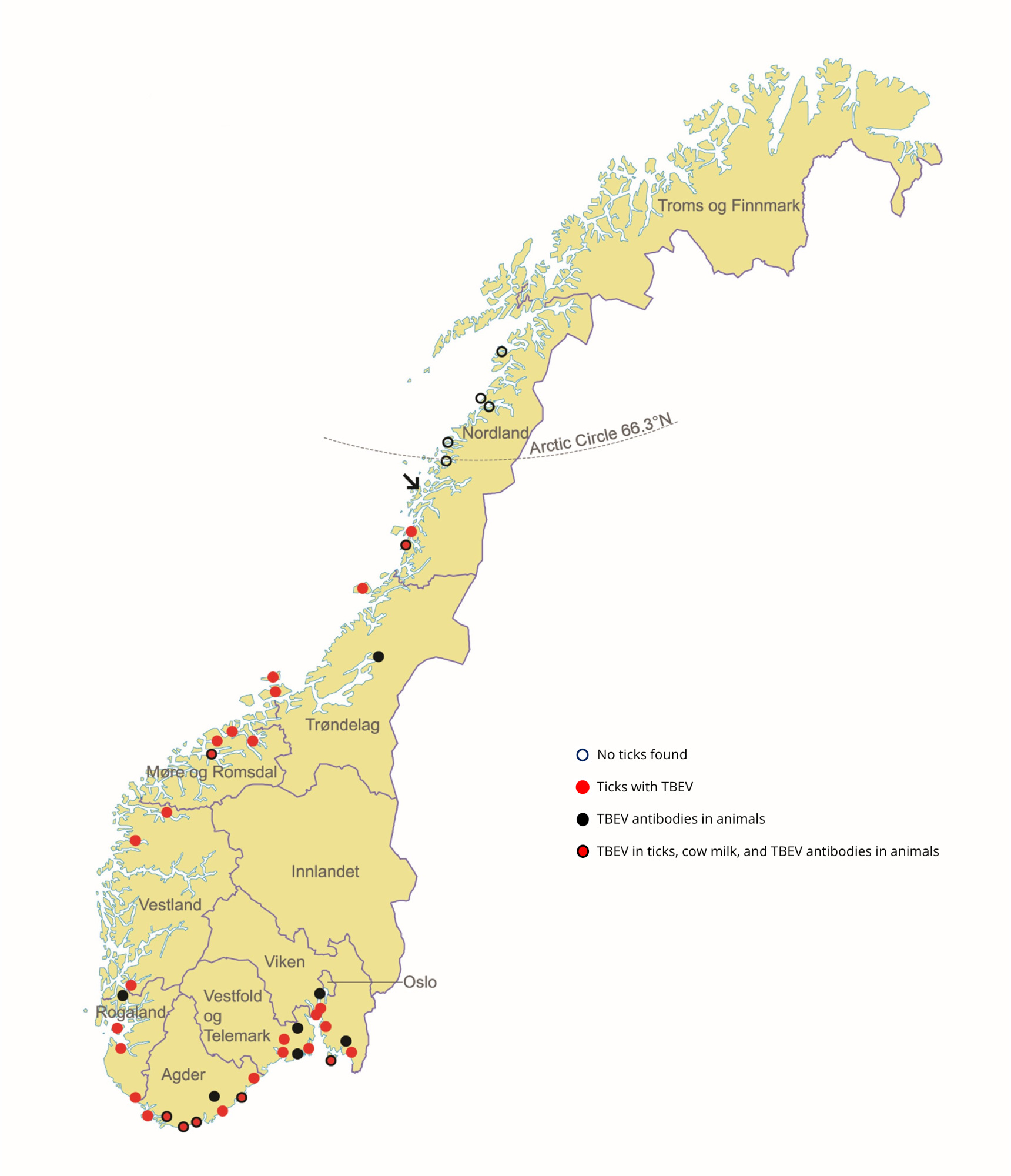
In addition, the first suggested isolate of TBEV in Norway was from I.ricinus ticks collected from the western coast of Norway.28 In the same area, antibodies against TBEV have been detected from human and bovine serum samples.32,36
Acknowledgements
We thank Trude Marie Lyngstad for making Figure 3.
Contact:
ashildkristine.andreassen@fhi.no
Citation:
Paulsen KM, Vikse R, Soleng A, Edgar KS, Lindstedt HH, Dorenberg DH, Wiklund BS, Andreassen KA. TBE in Norway. Chapter 12b. In: Dobler G, Erber W, Bröker M, Schmitt HJ, eds. The TBE Book. 6th ed. Singapore: Global Health Press; 2023. doi:10.33442/26613980_12b24-6
References
- Norwegian Surveillance System for Communicable Diseases (MSIS). 2021; Available from: www.MSIS.no, 02.21.
- Soleng A, et al. Distribution of Ixodes ricinus ticks and prevalence of tick-borne encephalitis virus among questing ticks in the Arctic Circle region of northern Norway. Ticks Tick Borne Dis. 2018;9(1):97-103.
- Paulsen KM, Pedersen BN, Soleng A, Okbaldet YB, Pettersson JH, Dudman SG, Ottesen P, Vik IS, Vainio K, Andreassen A. Prevalence of tick-borne encephalitis virus in Ixodes ricinus ticks from three islands in north-western Norway. APMIS. 2015;123(9):759-64.
- Paulsen KM, das Neves CG, Granquist EG, Madslien K, Stuen S, Pedersen BN, Vikse R, Rocchi M, Laming E, Stiasny K, Andreassen AK. Cervids as sentinel-species for tick-borne encephalitis virus in Norway – A serological study. Zoonoses Public Health. 2019. doi:10.1111/zph.12675.
- Paulsen KM, Stuen S, das Neves CG, Suhel F, Gurung D, Soleng A, Stiasny K, Vikse R, Andreassen AK, Granquist EG. Tick-borne encephalitis virus in cows and unpasteurized cow milk from Norway. Zoonoses Public Health. 2019;66(2):216-222.
- Vikse R, Paulsen KM, Edgar KS, Pettersson JH-O, Ottesen PS, Okbaldet YB, Kiran N, Lamsal A, Lindstedt HEH, Pedersen BN, Soleng A, Andreassen AK. Geographical distribution and prevalence of tick‐borne encephalitis virus in questing Ixodes ricinus ticks and phylogeographic structure of the Ixodes ricinus vector in Norway. Zoonoses Public Health. 2020:1-12. doi:10.1111/zph.12696.
- Kjelland V, Paulsen KM, Rollum R, Jenkins A, Stuen S, Soleng A, Edgar KS, Lindstedt HH, Vaino K, Gibory M, Andreassen AK. Tick-borne encephalitis virus, Borrelia burgdorferi sensu lato, Borrelia miyamotoi, Anaplasma phagocytophilum and Candidatus Neoehrlichia mikurensis in Ixodes ricinus ticks collected from recreational islands in southern Norway. Ticks Tick Borne Dis. 2018;9(5):1098-1102.
- Andreassen A, Jore S, Cuber P, Dudman S, Tengs T, Isaksen K, Hygen HO, Viljugrein H, Anestad G, Ottesen P, Vainio K. Prevalence of tick borne encephalitis virus in tick nymphs in relation to climatic factors on the southern coast of Norway. Parasit Vectors. 2012;5:177.
- Larsen AL, Kanestrom A, Bjorland M, Andreassen A, Soleng A, Vene S, Dudman SG. Detection of specific IgG antibodies in blood donors and tick-borne encephalitis virus in ticks within a non-endemic area in southeast Norway. Scand J Infect Dis. 2014;46(3):181-4.
- Skarpaas T, Ljøstad U, Sundøy A. First human cases of tickborne encephalitis, Norway. Emerg Infect Dis. 2004;10(12):2241-3.
- Skarpaas T, Golovljova I, Vene S, Ljøstad U, Sjursen H, Plyusnin A, Lundkvist A. Tickborne encephalitis virus, Norway and Denmark. Emerg Infect Dis. 2006;12(7):1136-8.
- Skarpaas T, et al. [Tick-borne encephalitis in Norway]. Tidsskr Nor Laegeforen. 2002;122(1):30-2.
- Csángó PA, Blakstad E, Kirtz GC, Pedersen JE, Czettel B. Tick-borne encephalitis in southern Norway. Emerg Infect Dis. 2004;10(3):533-4.
- Radzisauskiene D, Zagminas K, Asokliene L, Jasionis A, Mameniskiene R, Ambrozaitis A, Jancoriene L, Jatuzis D, IPetraityte I, Mockiene E. Epidemiological patterns of tick-borne encephalitis in Lithuania and clinical features in adults in the light of the high incidence in recent years: a retrospective study. Eur J Neurol. 2018;25(2):268-74.
- Kaminski M, Grummel V, Hoffmann D, Berthele A, Hemmer B. The spectrum of aseptic central nervous system infections in southern Germany – demographic, clinical and laboratory findings. Eur J Neurol. 2017;24(8):1062-70.
- Chitimia-Dobler L, et al. Repeated isolation of tick-borne encephalitis virus from adult Dermacentor reticulatus ticks in an endemic area in Germany. Parasit Vectors. 2019;12(1):90.
- Suss J. Epidemiology and ecology of TBE relevant to the production of effective vaccines. Vaccine. 2003;21 Suppl 1:S19-35.
- Rizzoli A, et al. Ixodes ricinus and Its Transmitted Pathogens in Urban and Peri-Urban Areas in Europe: New Hazards and Relevance for Public Health. Front Public Health. 2014;2:251.
- Tambs-Lyche. Ixodes ricinus og piroplasmosen i Norge (Meddelelse fra Bergen Museums zoologiske avdeling). 1943;55:337-366.
- Mehl R. The distribution and host relations of Norwegian ticks (Acari, Ixodides). Fauna norwgica series B. 1983;30:46-51.
- Hvidsten D, Frafjord K, Gray JS, Henningsson A, Jenkins A, Kristiansen BE, Lager M, Rognerud B, Slåtsve AM, Stordal F, Stuen S, Wilhelmsson P. The distribution limit of the common tick, Ixodes ricinus, and some associated pathogens in north-western Europe. Ticks Tick Borne Dis. 2020;3:101388.
- Jenkins A, Hvidsten D, Matussek A, Lindgren PE, Stuen S, Kristiansen BE. Borrelia burgdorferi sensu lato in Ixodes ricinus ticks from Norway: evaluation of a PCR test targeting the chromosomal flaB gene. Exp Appl Acarol. 2012; 58(4):431-9.
- Hvidsten D, Stuen S, Jenkins A, Dienus O, Olsen RS, Kristiansen BE, Mehl R, Matussek A. Ixodes ricinus and Borrelia prevalence at the Arctic Circle in Norway. Ticks Tick Borne Dis. 2014;5(2):107-12.
- Jore S, et al. Multi-source analysis reveals latitudinal and altitudinal shifts in range of Ixodes ricinus at its northern distribution limit. Parasit Vectors. 2011;4:84.
- Anonymous. Consensus conference on Lyme disease. Cmaj. 1991;144(12):1627-32.
- Piesman J. Field studies on Lyme disease in North America. Can J Infect Dis. 1991;2(2):55-7.
- Kjaer LJ, et al. A large-scale screening for the taiga tick, Ixodes persulcatus, and the meadow tick, Dermacentor reticulatus, in southern Scandinavia, 2016. Parasit Vectors. 2019;12(1):338.
- Traavik T, Mehl R, Wiger R. The first tick-borne encephalitis virus isolates from Norway. Acta Pathol Microbiol Scand B. 1978;86(4):253-5.
- Asghar N, Lindblom P, Melik W, Lindqvist R, Haglund M, Forsberg P, Overby AK, Andreassen A, Lindgren PE, Johansson M. Tick-borne encephalitis virus sequenced directly from questing and blood-feeding ticks reveals quasispecies variance. PLoS One. 2014;9(7):e103264.
- Ytrehus B, Vainio K, Dudman SG, Gilray J, Willoughby K. Tick-borne encephalitis virus and louping-ill virus may co-circulate in Southern Norway. Vector Borne Zoonotic Dis. 2013;13(10):762-8.
- Ytrehus B, et al. Louping-Ill Virus Serosurvey of Willow Ptarmigan (Lagopus lagopus lagopus) in Norway. J Wildl Dis. 2021;57(2):282-291. doi:10.7589/JWD-D-20-00068.
- Traavik T. Serological investigations indicating the existence of tick-borne encephalitis virus foci along the Norwegian coast. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973;81(1):138-42.
- Thortveit ET, Aase A, Petersen LB, Lorentzen ÅR, Mygland Å, Ljøstad U.Human seroprevalence of antibodies to tick-borne microbes in southern Norway. Ticks Tick Borne Dis. 2020;11(4):101410. doi: 10.1016/j.ttbdis.2020.101410.
- Marvik Å, et al. Low prevalence of tick-borne encephalitis virus antibodies in Norwegian blood donors. Infect Dis (Lond). 2021;53(1):44-51.
- Hjetland R, Henningsson AJ, Vainio K, Dudman SG, Grude N, Ulvestad E. Seroprevalence of antibodies to tick-borne encephalitis virus and Anaplasma phagocytophilum in healthy adults from western Norway. Infect Dis (Lond). 2015;47(1):52-6.
- Traavik T. Antibodies to tick-borne encephalitis virus in human sera from the western coast of Norway. Acta Pathol Microbiol Scand B. 1979;87B(1):9-13.
- Jaenson TG, Hjertqvist M, Bergstrom T, Lundkvist A. Why is tick-borne encephalitis increasing? A review of the key factors causing the increasing incidence of human TBE in Sweden. Parasit Vectors. 2012;5:184.
- Paulsen KM, et al. Experimental infection of lambs with tick-borne encephalitis virus and co-infection with Anaplasma phagocytophilum. PLoS One. 2019;14(12):e0226836.
- Paulsen KM, et al. High-throughput sequencing of two European strains of tick-borne encephalitis virus (TBEV), Hochosterwitz and 1993/783. Ticks Tick Borne Dis. 2021;12(1):101557.