Åke Lundkvist
E-CDC risk status: endemic
(data as of end 2022)
History and current situation
Tick-borne encephalitis virus (TBEV) was isolated for the first time in Sweden in 1958 (from ticks and from 1 tick-borne encephalitis [TBE] patient).1 In 2003, Haglund and colleagues reported the isolation and antigenic and genetic characterization of 14 TBEV strains from Swedish patients (samples collected 1991–1994).2 The first serum sample, from which TBEV was isolated, was obtained 2–10 days after onset of disease and found to be negative for anti-TBEV immunoglobulin M (IgM) by enzyme-linked immunosorbent assay (ELISA), whereas TBEV-specific IgM (and TBEV-specific immunoglobulin G/cerebrospinal fluid [IgG/CSF] activity) was demonstrated in later serum samples taken during the second phase of the disease.
Of 20 patient serum samples inoculated into the brain of suckling mice, 14 induced obvious signs of illness (death or clear physical signs in all cases, 5–7 days after inoculation), and TBEV was isolated in all cases. Three earlier Swedish TBEV patient isolates from 1958,1 1959, and 1966, respectively, were included in the same study. Phylogenetic analyses of the partial sequence (domain III) of the E gene revealed that all Swedish TBEV strains grouped together with the previously characterized strains (Neudoerfl, Kumlinge-A52, Hypr, and TBE 263) of the Western or European subtype of TBEV (TBEV-EU).
In 2007, a partial TBEV sequence (approximately one-third of the viral genome) from a small pool of ticks collected in the Stockholm archipelago on the island of Torö was reported.3
The sequence was characterized and compared with those of other tick-borne flaviviruses, which led to classification of the virus as TBEV-EU. The same group reported in 2011 on the first complete genome of a Swedish TBEV strain by completing the earlier partial sequencing (see above).4 The total RNA was sufficient for the sequencing of a complete TBEV genome (Torö-2003), without conventional enrichment procedures such as cell culture or amplification in suckling mice. Sequence analyses also revealed that Torö-2003 belonged to the TBEV-EU subtype, being most similar to TBE 263 with 97.4% and 98.8% homologies at the nucleotide and amino acid levels, respectively.
In 2014, Veje and coworkers reported 2 cases of TBE in which TBEV RNA could be detected in urine by real-time polymerase chain reaction (PCR) during the encephalitic phase.5 The TBEV RNA quantities from 1 patient allowed sequencing of 10,432 nucleotides (nt), which confirmed the PCR finding in urine, and phylogenetic analysis showed that the virus belonged to the TBEV-EU clade.
In 2016, Henningsson and associates reported isolation and a complete TBEV sequence from a biting tick.6 By performing nt sequencing of the virus strain (Tick/SWE/Habo/2011/1) via 2 different strategies (deep sequencing of the A549 isolate and direct sequencing of PCR amplicons of RNA extracted from the tick, respectively), the authors showed that the 2 sequences were identical over 3382 nt, thereby suggesting that the virus isolation procedure did not introduce a selection bias with regard to the compared nt sequences.
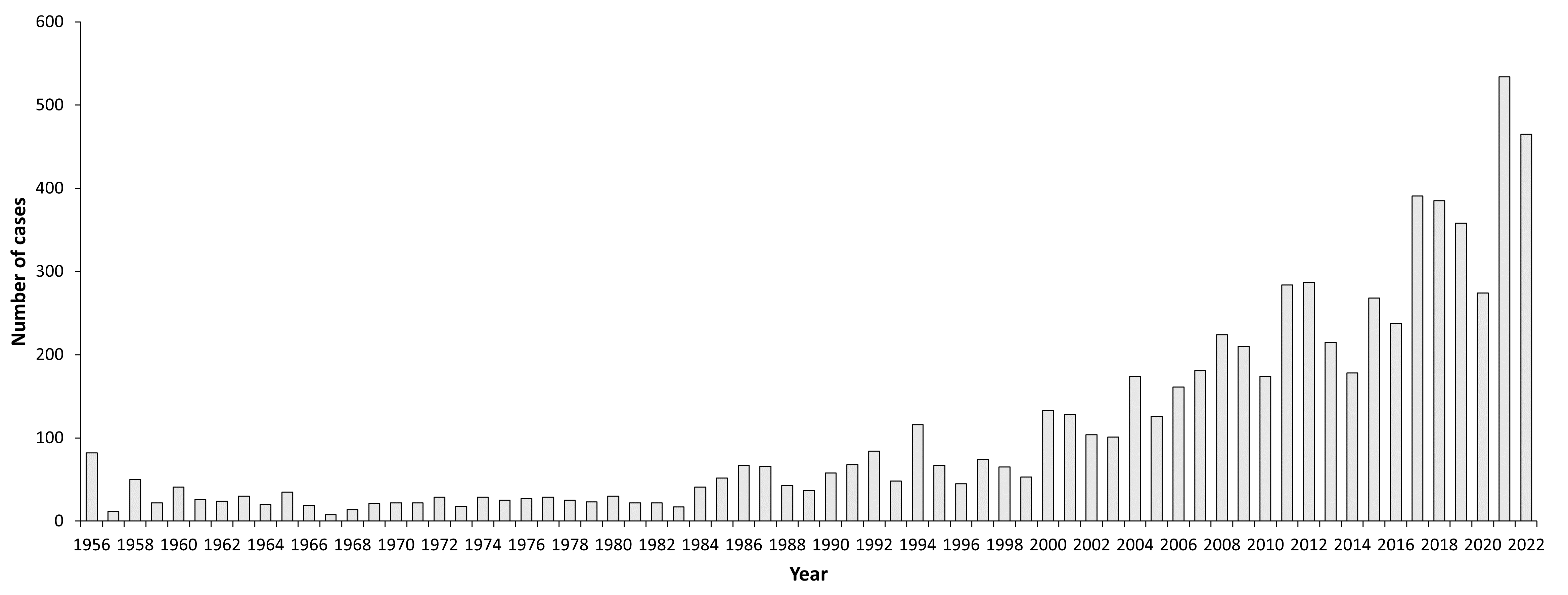
As in other areas of Europe, the number of reported TBE cases has increased during the last 25 years. The mortality of TBE in Sweden is significant (1.4%)7 and the morbidity and long-term sequelae make it a disease of great importance in the endemic regions.8–10 TBE has been reported in Sweden from diagnostic laboratories on a voluntary basis since the 1970s and notification has been mandatory since 2004. During the years 2007–2019, between 181 and 391 (year 2017) cases of TBE were reported annually in Sweden despite the fact that vaccination has increased in the exposed population. There are 2 TBE vaccines available in Sweden: FSME-Immun (Pfizer) introduced in 1988 and Encepur (Bavarian Nordic) introduced in 2003.
Vaccination against TBE is voluntary in Sweden. The vaccination schedule recommended in Sweden follows the recommendations of the manufacturers, with one exception being that after dose 4 and onwards, a 5-year interval is recommended, irrespective of age (the manufacturers recommend 3-year booster intervals after the age of 60). The change to a 5-year interval after dose 4 and onwards was based on a large study of the serological response in 535 persons in Sweden after TBE vaccination.11 However, if TBE vaccination is initiated over age 60, the recommended schedule is 1 extra dose 2 months after the second dose, i.e. the initial vaccination includes 4 doses at 0, 1, 3, and 5-12 months.
The number of vaccine doses sold in Sweden has averaged from 500,000 to 600,000 annually since 2006, but increased to 1.2 million doses per year in 2018. Because TBE vaccination is not included in any official vaccination registry, the actual number of immunized individuals is unknown.
To estimate the TBE vaccination coverage in the greater Stockholm region, a questionnaire was sent to a randomized sample of 8000 individuals in 2013.12 Fifty-three percent of all respondents reported being vaccinated against TBE at least once. Based on these findings, the estimated TBE incidence in the unvaccinated regional population was 8.5–12/100,000, which is comparable to highly endemic areas in the Baltics and Central Europe.
The protection rate of the vaccine has been estimated to be 96% to 98% according to field studies in Austria. In a study from 2010, data from 27 Swedish patients with clinical symptoms and signs of TBE, together with serological evidence of TBEV infection despite active vaccination, was presented.13 These vaccination failures were characterized by a slow and initially non-detectable development of TBEV-specific IgM, seen together with a rapid rise of IgG and neutralizing antibodies in serum. The majority (70%) of the 27 patients were above age 50, which indicated the need for a modified immunization strategy in the elderly (as noted above).
Recently, a new tool (TBE suspension multiplex immunoassay, TBEV SMIA) for improved diagnostics of TBEV infections was reported.17 The TBEV SMIA can accurately differentiate TBEV infections from TBE vaccination and further studies have now been initiated to evaluate the efficiency of the assay for diagnosis of potential vaccine failures.
Recently, the TBEV SMIA was evaluated using samples from 14 previously confirmed Swedish TBEV vaccine failure patients.18 The conclusion was that detection of antibodies directed to TBEV NS1 antigen is a useful tool to considerably simplify and improve the quality in investigations regarding suspected TBEV infection in vaccinated patients.
In northern Europe, including Sweden, TBEV-EU is usually transmitted to humans by the common tick, Ixodes ricinus. Pettersson and colleagues investigated the prevalence in host-seeking I. ricinus in southern and central Sweden and reviewed all relevant published records on the prevalence of TBEV in ticks in northern Europe.14 The estimated mean minimum infection rate (MIR) of TBEV in nymphal and adult I. ricinus for northern Europe (i.e., Denmark, Norway, Sweden, and Finland) was 0.28% and 0.23% for southern Sweden. Also, the infection prevalence of TBEV was significantly lower for nymphs (0.10%) than in adult ticks (0.55%). At a well-known TBEV-endemic region, Torö island, southeast of Stockholm, the TBEV prevalence was 0.51% in nymphs and 4.48% in adult ticks.
In a review of the ecology and epidemiology of TBE in Sweden, Jaenson and colleagues analyzed the possible reasons behind the gradually increasing incidence of human TBE during the last 20 years.15 The authors made the following conclusions:
- Due to a large roe deer population during the 1980s and 1990s, the Swedish tick population gradually increased. At the turn of the century, the tick population in Sweden was probably larger than ever.
- The roe deer population gradually declined after its peak in the late 1980s and early 1990s.
- During the decline of the roe deer population, a gradually larger proportion of the tick larvae and nymphs probably fed on small mammals, which are reservoir-competent hosts for TBEV. Consequently, since the mid-1990s, a larger proportion of the tick population became infected with TBEV.
- Climate change and weather events associated with higher temperatures further influenced the infection prevalence in the tick population and therefore also the annual incidence in humans.
Overview of TBE in Sweden
| Table 1: Virus, vector, transmission of TBE in Sweden | |
|---|---|
| Viral subtypes, distribution | Only western/European TBEV (TBEV-EU), southern part of the country1-6 |
| Reservoir animals | Not documented |
| Infected tick species (%) | Ixodes ricinus. 0.23% to 4.48%14 |
| Dairy product transmission | Not documented |
Figure 1: Burden of TBE in Sweden over time
| Year | Number of cases | Incidence / 105 |
|---|---|---|
| 1956 | 82 | 1.1 |
| 1957 | 12 | 0.16 |
| 1958 | 50 | 0.67 |
| 1959 | 22 | 0.29 |
| 1960 | 41 | 0.55 |
| 1961 | 26 | 0.34 |
| 1962 | 24 | 0.32 |
| 1963 | 30 | 0.39 |
| 1964 | 20 | 0.26 |
| 1965 | 35 | 0.45 |
| 1966 | 19 | 0.24 |
| 1967 | 8 | 0.1 |
| 1968 | 14 | 0.18 |
| 1969 | 21 | 0.26 |
| 1970 | 22 | 0.27 |
| 1971 | 22 | 0.27 |
| 1972 | 29 | 0.036 |
| 1973 | 18 | 0.22 |
| 1974 | 29 | 0.35 |
| 1975 | 25 | 0.3 |
| 1976 | 27 | 0.33 |
| 1977 | 29 | 0.35 |
| 1978 | 25 | 0.3 |
| 1979 | 23 | 0.28 |
| 1980 | 30 | 0.36 |
| 1981 | 22 | 0.26 |
| 1982 | 22 | 0.26 |
| 1983 | 17 | 0.2 |
| 1984 | 41 | 0.49 |
| 1985 | 52 | 0.63 |
| 1986 | 67 | 0.8 |
| 1987 | 66 | 0.78 |
| 1988 | 43 | 0.51 |
| 1989 | 37 | 0.43 |
| 1990 | 58 | 0.68 |
| 1991 | 68 | 0.79 |
| 1992 | 84 | 0.97 |
| 1993 | 48 | 0.55 |
| 1994 | 116 | 1.3 |
| 1995 | 67 | 0.76 |
| 1996 | 45 | 0.51 |
| 1997 | 74 | 0.84 |
| 1998 | 65 | 0.73 |
| 1999 | 53 | 0.6 |
| 2000 | 133 | 1.5 |
| 2001 | 128 | 1.4 |
| 2002 | 104 | 1.2 |
| 2003 | 101 | 1.1 |
| 2004 | 174 | 1.9 |
| 2005 | 126 | 1.4 |
| 2006 | 161 | 1.8 |
| 2007 | 181 | 2 |
| 2008 | 224 | 2.4 |
| 2009 | 210 | 2.2 |
| 2010 | 174 | 1.8 |
| 2011 | 284 | 3 |
| 2012 | 287 | 3 |
| 2013 | 209 | 2.17 |
| 2014 | 178 | 1.83 |
| 2015 | 268 | 2.72 |
| 2016 | 238 | 2.38 |
| 2017 | 391 | 3.86 |
| 2018 | 385 | 3.76 |
| 2019 | 358 | 3.47 |
| 2020 | 274 | 2.64 |
| 2021 | 534 | 5.11 |
| 2022 | 465 | 4.42 |
| Table 2: TBE reporting and vaccine prevention in Sweden | |
|---|---|
| Mandatory TBE reporting | Each diagnostic laboratory plus the responsible physician report to the Public Health Agency of Sweden
Case definition: TBEV-infection (viral TBE) Suspected case: – Epidemiological link – Clinical symptoms consistent with TBE – Pleocytosis (CSF) and/or neurological symptoms of encephalitis – Detection of TBEV-specific serum IgM Confirmed case: At least one of the following: – Detection of TBE-specific IgM and IgG in serum – Detection of TBE-specific IgM in CSF – Seroconversion or significant titer rise in paired serum samples – Detection of TBEV RNA in CSF (or post-mortem in brain tissue) – Detection of TBEV RNA in serum Note: Previous TBE vaccination and/or immunosuppression influence the patients’ antibody responses and thus repeated sampling may be necessary for an accurate diagnosis. Also earlier infections, or vaccinations, against other flaviviruses may complicate the diagnostics due to cross-reactive antibodies. Source: The Public Health Agency of Sweden (see below) |
| Other TBE surveillance | No |
| Clinical characteristics | 36%–40% with sequelae (after 1 year); Mortality: 1.4%7-8 |
| Available vaccines | FSME-Immun (Pfizer) introduced in 1988 and Encepur (Bavarian Nordic) introduced in 2003. 500,000–600,000 doses/year;13,16 1,200,000 doses/year in 2018 (unpublished data) |
| Vaccination recommendations and reimbursement | Revised each year
No reimbursement |
| Vaccine uptake by age group/risk group/general population | No data available |
| Name, address/website of TBE National Reference Center | The Public Health Agency of SwedenSE-171 82 Solna , Sweden
|
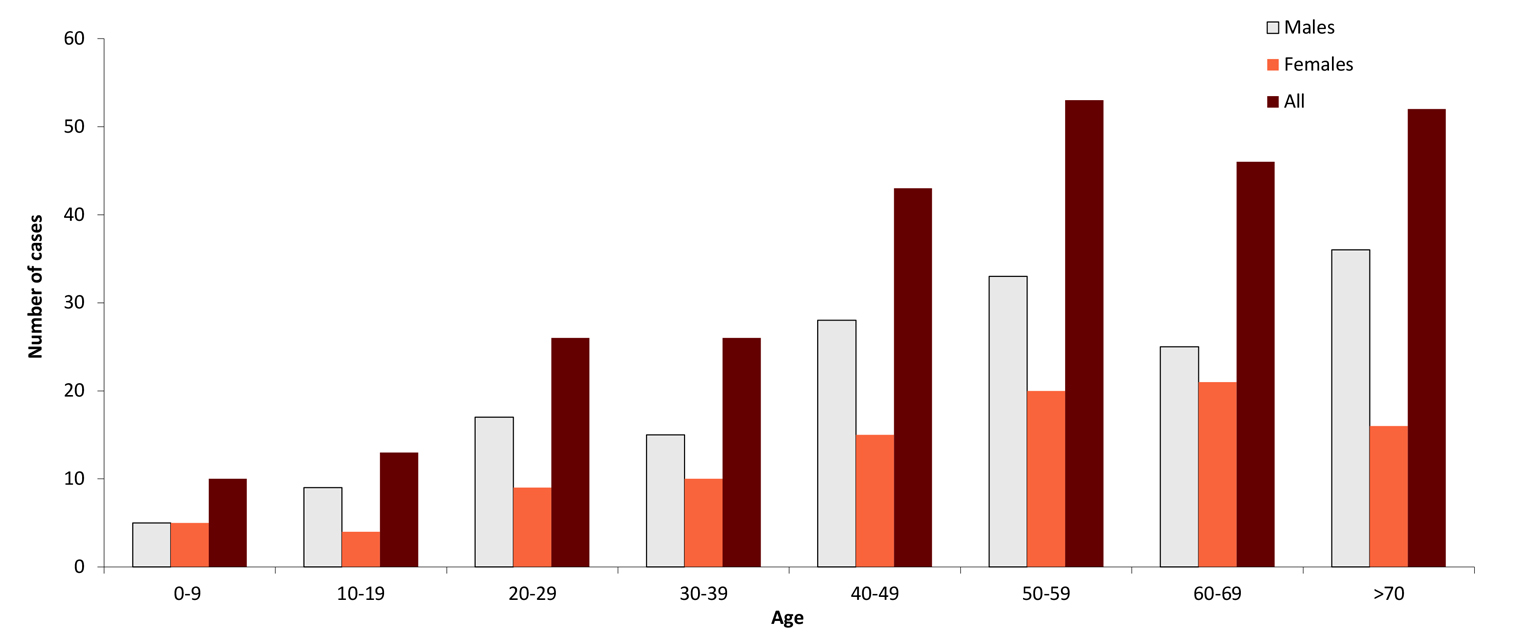
Figure 2: Age and gender distribution of TBE cases in Sweden (2018)
| Age group (years) | Males | Females | All |
|---|---|---|---|
| 0-9 | 5 | 5 | 10 |
| 10-19 | 9 | 4 | 13 |
| 20-29 | 17 | 9 | 26 |
| 30-39 | 15 | 10 | 25 |
| 40-49 | 28 | 15 | 43 |
| 50-59 | 33 | 20 | 53 |
| 60-69 | 25 | 21 | 46 |
| >70 | 36 | 16 | 52 |
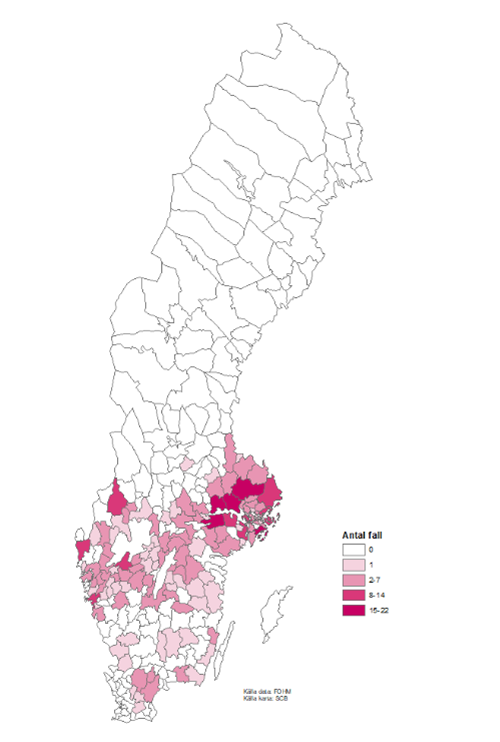
Figure 3: TBE cases per municipality in 2021
Contact:
ake.lundkvist@imbim.uu.se
Citation:
Lundkvist A. TBE in Sweden. Chapter 12b. In: Dobler G, Erber W, Bröker M, Schmitt HJS, eds. The TBE Book. 6th ed. Singapore: Global Health Press; 2023. doi: 10.33442/26613980_12b32-6
References
- Von Zeipel G. Isolation of viruses of the Russian Spring-Summer Encephalitis-Louping ill group from Swedish ticks and from a human case of meningo-encephalitis. Arch Gesamte Virusforsch. 1959;9:460-9.
- Haglund M, Vene S, Forsgren M, et al. Characterization of human tick-borne encephalitis virus from Sweden. J Med Virol. 2003;71:610-21.
- Melik W, Nilsson AS, Johansson M. Detection strategies of tick-borne encephalitis virus in Swedish Ixodes ricinus reveal evolutionary characteristics of emerging tick-borne flaviviruses. Arch Virol. 2007;152:1027-34.
- Elväng A, Melik W, Bertrand Y, Lönn M, Johansson M. Sequencing of a tick-borne encephalitis virus from Ixodes ricinus reveals a thermosensitive RNA switch significant for virus propagation in ectothermic arthropods. Vector Borne Zoonotic Dis. 2011;11:649-58.
- Veje M, Studahl M, Norberg P, et al. Detection of tick-borne encephalitis virus RNA in urine. J Clin Microbiol. 2014;52:4111-2.
- Henningsson AJ, Lindqvist R, Norberg P, et al. Human tick-borne encephalitis and characterization of virus from biting tick. Emerg Infect Dis. 2016;22:1485-7.
- Haglund M, Forsgren M, Lindh G, Lindquist L. A 10-year follow-up study of tick-borne encephalitis in the Stockholm area and a review of the literature: need for a vaccination strategy. Scand J Infect Dis. 1996;28:217-24
- Günther G, Haglund M, Lindquist L, Forsgren M, Sköldenberg B. Tick-borne encephalitis in Sweden in relation to aseptic mening-encephalitis of other etiology: a prospective study of clinical course and outcome. J Neurol. 1997;244:230-38.
- Haglund M, Günther G. Tick-borne encephalitis – pathogenesis, clinical course and long-term follow-up. Vaccine. 2003;21 Suppl 1:11-8.
- Lindquist L, Vapalahti O. Tick-borne encephalitis. Lancet. 2008;371(9627):1861-71.
- Vene S, Haglund M, Lundkvist Å, Lindquist L, Forsgren M. Study of the serological response after vaccination against tick-borne encephalitis in Sweden. Vaccine. 2007;25(2):366-72.
- Askling HH, Insulander M, Hergens MP, Leval A. Tick-borne encephalitis (TBE)-vaccination coverage and analysis of variables associated with vaccination, Sweden. Vaccine. 2015;33(38):4962-8.
- Andersson C, Vene S, Insulander M, et al. Vaccine failures after active immunization against tick-borne encephalitis. Vaccine. 2010;28(16):2827-31.
- Pettersson JH, Golovljova I, Vene S, Jaenson TG. Prevalence of tick-borne encephalitis virus in Ixodes ricinus ticks in northern Europe with particular reference to Southern Sweden. Parasit Vectors. 2014;7:102.
- Jaensson TG, Hjertqvist M, Bergström T, Lundkvist Å. Why is tick-borne encephalitis increasing? A review of the key factors causing the increasing incidence of human TBE in Sweden. Parasit Vectors. 2012;5:184.
- Slunge D. The willingness to pay for vaccination against tick-borne encephalitis and implications for public health policy: Evidence from Sweden. PLoS One. 2015;10:e0143875.
- Albinsson B, Vene S, Rombo L, Blomberg J, Lundkvist Å, Rönnberg B Distinction between serological responses following tick-borne encephalitis virus (TBEV) infection vs vaccination, Sweden 2017. Euro Surveill. 2018;23(3).
- Albinsson B, Rönnberg B, Vene S, Lundkvist Å. Antibody responses to tick-borne encephalitis virus non-structural protein 1 and whole virus antigen-a new tool in the assessment of suspected vaccine failure patients. Infect Ecol Epidemiol. 2019;9(1):1696132.