Katarzyna Pancer and Włodzimierz Gut
E-CDC risk status: endemic
(data as of end 2022)
History and current situation
Clinical symptoms of tick-borne encephalitis (TBE) were first described in Poland in 1948 by Demiaszkiewicz. All patients had been living in the Białowieża region (in northeastern Poland). Similar infections were described to those that had been diagnosed in the same region before World War II as complicated cases of typhoid fever or influenza.1
Twenty-eight cases of TBE were identified in 1952 among patients hospitalized in Nysa Kłodzka (in southwestern Poland). In 1954, 35 cases were identified in the Olsztyn region in northern Poland. More cases were recognized in the following years across different regions of Poland: northern (Gdańsk, Szczecin), central (Łódź), and southern (Kraków). This was the catalyst for scientific research studies by Przesmycki’s team in selected regions of the country in the years 1953 through 1957.2 In these studies, tick-borne encephalitis viruses (TBEV) were isolated from human specimens as well as from animal samples (small mammals) and vectors (ticks). Isolated viruses were determined to be TBEV, European subtype.2–4
Seroprevalence studies were conducted in the late 1960s and early 1970s. Serum samples collected from ~17,000 blood donors and 20,000 forest workers living in different parts of Poland were examined. Distribution of positive serological results varied depending on place of residence: ranging from 0.5% to 6.5% among blood donors and 7% to 27% among forest workers. The seroprevalence data also indicated high numbers of asymptomatic or non-severe infections among examined populations.2,5–9
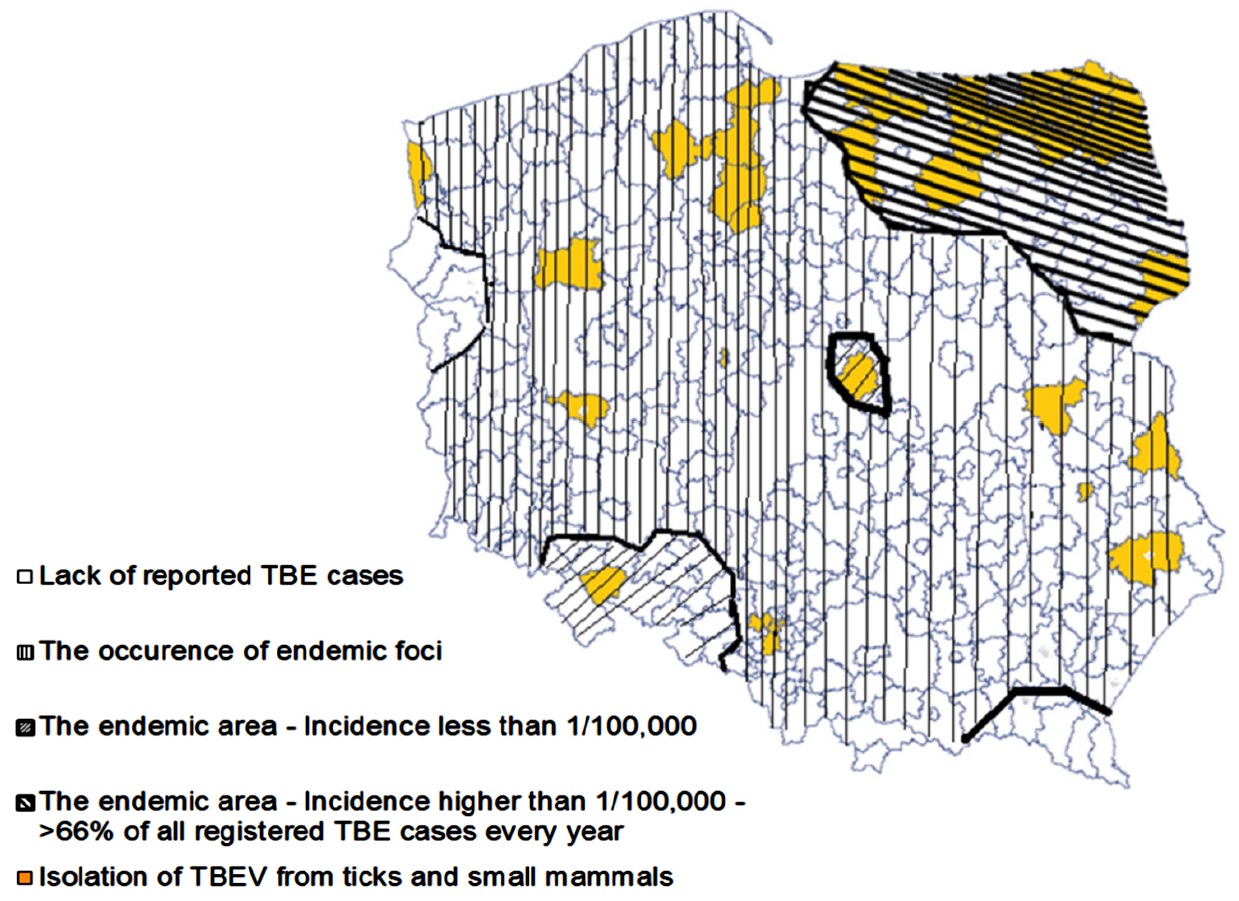
The developed distribution map of TBE cases and confirmed presence of TBEV in Poland was based on results from seroprevalence studies, viral isolation, and clinical data. Some regions were determined to be endemic for TBEV. These included provinces in the northeastern part of Poland (Białystok, Olsztyn, Suwalki) and southwestern Poland (Opole).3,4,6–12
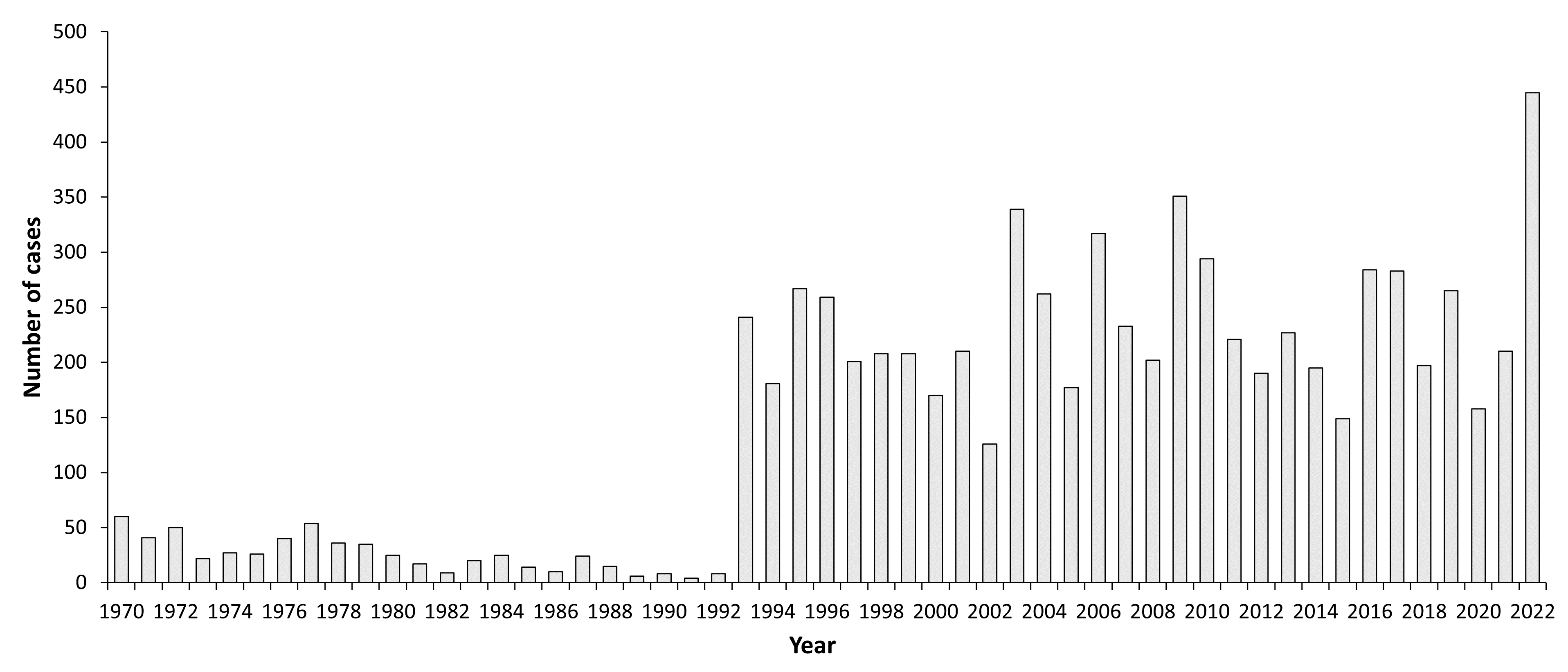
In total, 576 TBE cases were reported during the 23 years of surveillance (1970–1992); the annual number of reported TBE infections varied from 4 (1991) to 60 (1970), and the incidence ranged from 0.01/100,000 inhabitants to 0.2/100,000 inhabitants, respectively. In the 1980s, the number of reported TBE cases decreased to 14–19 cases annually because of abandonment of diagnostics tests.2,13
In 1993, when new commercial tests became available in Poland, reported TBE cases increased more than 30-fold in comparison to 1992 (249 vs. 8 cases). In 1993, the incidence of TBE (0.65/100,000) was the highest observed since surveillance began in 1970. This trend continued into the 21st century, and more than 300 TBE cases were reported in the years 2003 (339 cases), 2006 (317 cases), and 2009 (351 cases). The highest incidence (0.92/100,000) was reported in 2009. The annual number of reported TBE cases decreased to 149 in 2015.13
In total, 3,662 cases of TBE were reported in Poland between 2000 and 2015. The incidence has varied from 0.33 to 0.92/100,000. A 3–4-year cycle was identified based on the reported numbers of TBE cases, with peaks observed in 2003, 2006, and 2009. TBE cases were identified in all regions of Poland except one: there was no diagnosed or reported TBE case in Lubuskie Province, which is located at the Western border region along the banks of the Odra River. In contrast, more than 70% of the reported cases in each year were diagnosed in two provinces in the northeastern part of Poland: Podlaskie (Białystok) with >45% reported TBE cases and an incidence >6/100,000, and Warmińsko-Mazurskie (Olsztyn) with 25% cases and an incidence >1.5/100,000. Also, outbreaks of TBE were observed in those same regions during spring-summer time.13
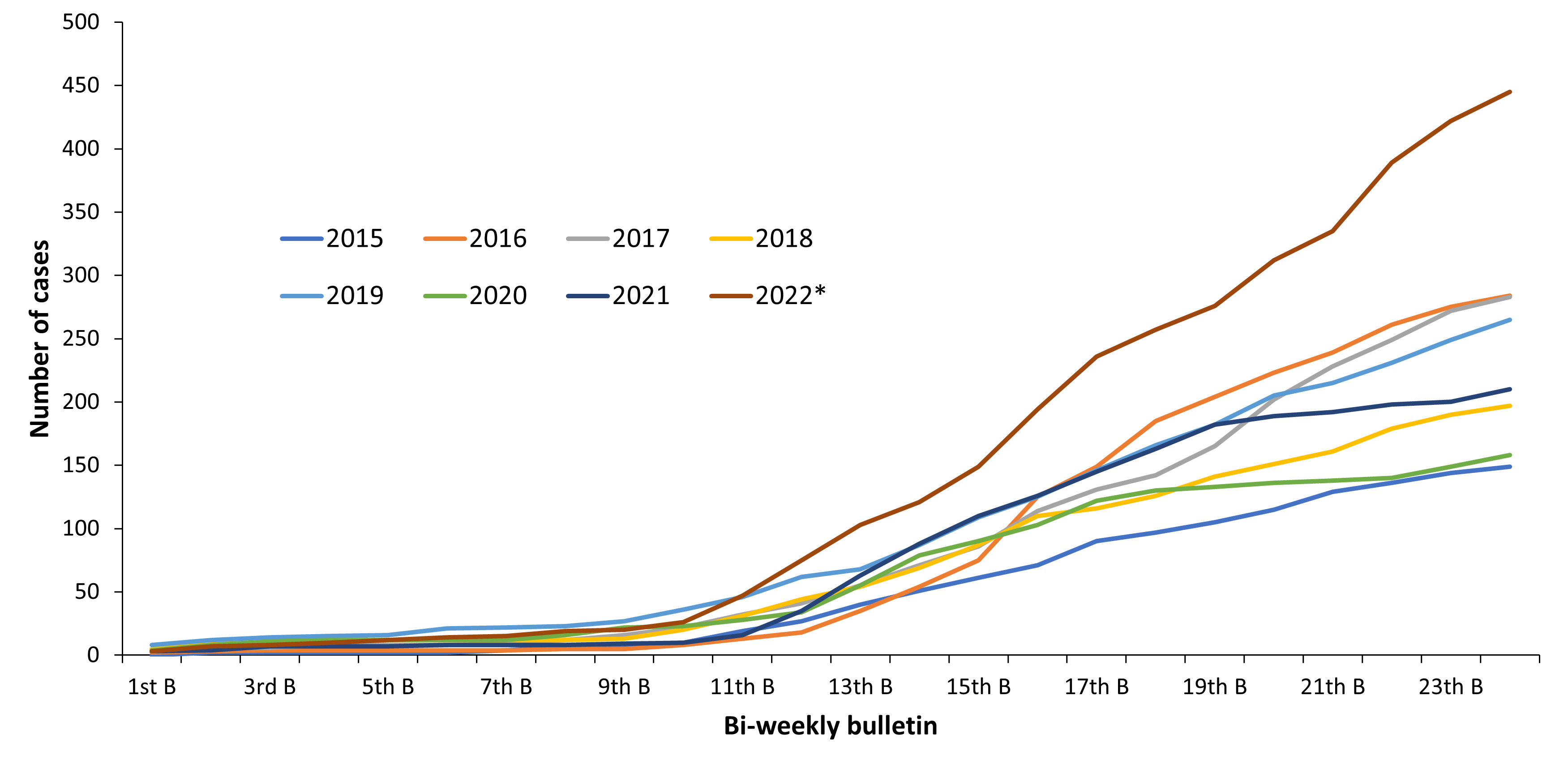
In contrast to Central European countries (Germany, Czech Republic, Austria, Switzerland) the reported Polish TBE case numbers in 2018 did not significantly increase in summer time. Also the total number of 197 TBE cases is ~30% lower than in the previous years (279 cases in 2017, 283 cases in 2016) (Fig.3). However, a similar phenomenon of increased number of the reported TBE cases during the summer time was observed in 2016, but the total number of TBE cases this year was comparable to those reported in 2017 but higher than the number of TBE cases reported in 2015 (149 cases)20.
The age of patients with diagnosed TBE ranged from 3 to 80 years, but the majority of patients were >20 years old.13 Almost 20% of all reported TBE cases were associated with work or visits in the area where TBEV-infected ticks were found. Moreover, food-borne transmission was documented in 1975 and 1995. The source of infection was fresh, non-pasteurized milk of cows (1975) or goats (1995) contaminated with TBEV.14,15
The mortality rate observed for the reported TBE cases in Poland ranged from 0.5% to 2.8% and was similar to that observed in other European countries where European subtype of TBEV (TBEV-EU) variants have been confirmed.5,13
Prevention of TBE is based on decreasing the probability of infection by limiting exposure to infected ticks (wearing appropriate clothing, use of insect repellents, etc.), by vaccination, and by appropriate preparation of milk (pasteurization, boiling). Since 1952 the commercial sale of milk in Poland is only allowed after thermal preparation. However, fresh milk is still available in local markets.14,15
In Poland vaccination against TBEV started in the 1970s. At the beginning of this campaign, vaccination was done using the Russian (local brand name: “Vaccinum Encephalitis Ixodice”), which consisted of a formalin-inactivated TBEV-Siberian type. Since 1993 this vaccine was replaced by the two EMA-licensed vaccines with a TBEV-EU subtype as the seed virus for production (FSME-Immun (Pfizer) and Encepur (Bavarian Nordic)).2,16–17 Both vaccines are available for use in children and adults. Vaccination against TBEV is recommended in Poland, especially for forest workers, foragers of forest undergrowth, and tourists. The costs of vaccination are not reimbursed, except through campaigns paid for by employers or local communities (medical service, forest workers etc.). In Poland, 27,849 persons were vaccinated in 2015, among them 11,516 below the age of 19 years.18 The rather low rate of vaccination against TBE among people in Poland has no effect on the number of reported TBE cases and epidemiological characteristics of TBEV infection.
Overview of TBE in Poland
| Table 1: Virus, vector, transmission of TBE in Poland | |
|---|---|
| Viral subtypes, distribution | European subtype (also called Western European or Central European subtype) |
| Reservoir animals | Rodents, Tick2,7 |
| Infected tick species (%) | Ixodes ricinus, depending on region and used technique, range of Minimum Infection Rate from 0.00 to 1.963,4,7,10-12 |
| Dairy product transmission | Rare (1975; 1995)14,15 |
| Table 2: TBE reporting and vaccine prevention in Poland | |
|---|---|
| Mandatory TBE reporting | ONLY cases of neuroinfection Case definition—per ECDC (2.44)19 Clinical Criteria: Any person with symptoms of inflammation of the central nervous system (CNS): e.g. meningitis, meningo-encephalitis, encephalomyelitis, encephalo-radiculitisLaboratory Criteria: Laboratory criteria for case confirmation At least 1 of the following 5 criteria:
Laboratory criteria for a probable case:
Epidemiological Criteria: Exposure to a common source (unpasteurized dairy products) Case Classification
|
| Other TBE-surveillance | Obligatory reporting by diagnostic laboratory of any positive results from serological (IgM) examination to local health service in the patient’s place of residence |
| Special clinical features | Contact with ticks; consumption of non-pasteurized dairy products14,15 Mortality 0.5%–2.8%13 |
| Available vaccines | Since 1993:
|
| Vaccination recommendations and reimbursement | Recommendation for additional supplementary immunization – 1970s. No reimbursement* |
| Vaccine uptake by age group/ risk group/general population | In 2015, 27,849 persons; among them 11,516 who are <20 years of age18 |
| Name, address/website of TBE National Reference Center | Lack of Reference Laboratory or Center – since 2004 (due to more stable/constant disease situation) |
*In Poland, vaccination against TBE is recommended (but not financed from the budget of the Ministry of Health) for persons in areas with severe occurrence of the disease, in particular:
- forest workers
- foragers (e.g., persons who harvest mushrooms, berries, etc. – commercially or recreationally)
- stationed military
- guards brigade and border
- farmers
- young people in practice (outdoor play and recreation)
- tourists and visitors to camps and colonies
| Year | Number of Cases | Incidence / 105 |
|---|---|---|
| 1970a | 60 | 0.15 |
| 1971 | 41 | 0.10 |
| 1972 | 50 | 0.125 |
| 1973 | 22 | 0.05 |
| 1974 | 27 | 0.07 |
| 1975b | 26 | 0.07 |
| 1976 | 40 | 0.10 |
| 1977 | 54 | 0.14 |
| 1978 | 36 | 0.10 |
| 1979 | 35 | 0.09 |
| 1980 | 25 | 0.06 |
| 1981 | 17 | 0.04 |
| 1982 | 9 | 0.007 |
| 1983 | 20 | 0.045 |
| 1984 | 25 | 0.05 |
| 1985# | 14 | 0.03 |
| 1986 | 10 | 0.02 |
| 1987 | 24 | 0.06 |
| 1988 | 15 | 0.03 |
| 1989 | 6 | 0.04 |
| 1990 | 8 | 0.006 |
| 1991 | 4 | 0.003 |
| 1992 | 8 | 0.006 |
| 1993c | 241 | 0.63 |
| 1994 | 181 | 0.47 |
| 1995 | 267 | 0.70 |
| 1996 | 259 | 0.69 |
| 1997 | 201 | 0.53 |
| 1998 | 208 | 0.54 |
| 1999 | 208 | 0.54 |
| 2000 | 170 | 0.44 |
| 2001 | 210 | 0.54 |
| 2002 | 126 | 0.33 |
| 2003d | 339 | 0.89 |
| 2004 | 262 | 0.69 |
| 2005 | 177 | 0.46 |
| 2006 | 317 | 0.83 |
| 2007 | 233 | 0.61 |
| 2008 | 202 | 0.53 |
| 2009 | 351 | 0.92 |
| 2010 | 294 | 0.77 |
| 2011 | 221 | 0.57 |
| 2012 | 190 | 0.49 |
| 2013 | 227 | 0.59 |
| 2014 | 195 | 0.51 |
| 2015 | 149 | 0.39 |
| 2016 | 284 | 0.74 |
| 2017 | 283 | 0.74 |
| 2018 | 197 | 0.51 |
| 2019 | 265 | 0.69 |
| 2020 | 158 | 0.42 |
| 2021 | 210 | 0.56 |
| 2022e | 445 | 1.18 |
b1975: Establishment of National Arbovirus Laboratory, National Institute of Public Health – National Institute of Hygiene (NIPH-NIH) and production of hemagglutination inhibition (HI) antigen for surveillance service to the end of 1984
cDiagnostics based on ELISA method in hospital and Sanitary Service laboratories with confirmation in Reference Laboratory NIH; 1993–2003 recommended vaccination against TBEV-EU (not reimbursed)
dLack of reference laboratory because of expiry of the mandate and law regulation – from that time there is no necessity to confirm positive serological results for TBE
eData for 2022 is not verified
#From 1970 to 1985 confirmation based on HI test; since 1993, IgM ELISA for confirmation (and local synthesis of TBEV-specific IgG in CSF)
1970: Start of registration of TBE in Poland; 1970–1984 recommended vaccination with Russian anti-TBEV Siberian type (not reimbursed)
1975: Establishment of National Arbovirus Laboratory, National Institute of Public Health – National Institute of Hygiene (NIPH-NIH) and production of hemagglutination inhibition (HI) antigen for surveillance service to the end of 1984
Diagnostics based on ELISA method in hospital and Sanitary Service laboratories with confirmation in Reference Laboratory NIH; 1993–2003 recommended vaccination against TBEV-EU (not reimbursed)
Lack of reference laboratory because of expiry of the mandate and law regulation – from that time there is no necessity to confirm positive serological results for TBEV
From 1970 to 1985 confirmation based on HI test; since 1993, IgM ELISA for confirmation (and local synthesis of TBEV-specific IgG in CSF)
Figure 2: Age and gender distribution of TBE in Poland
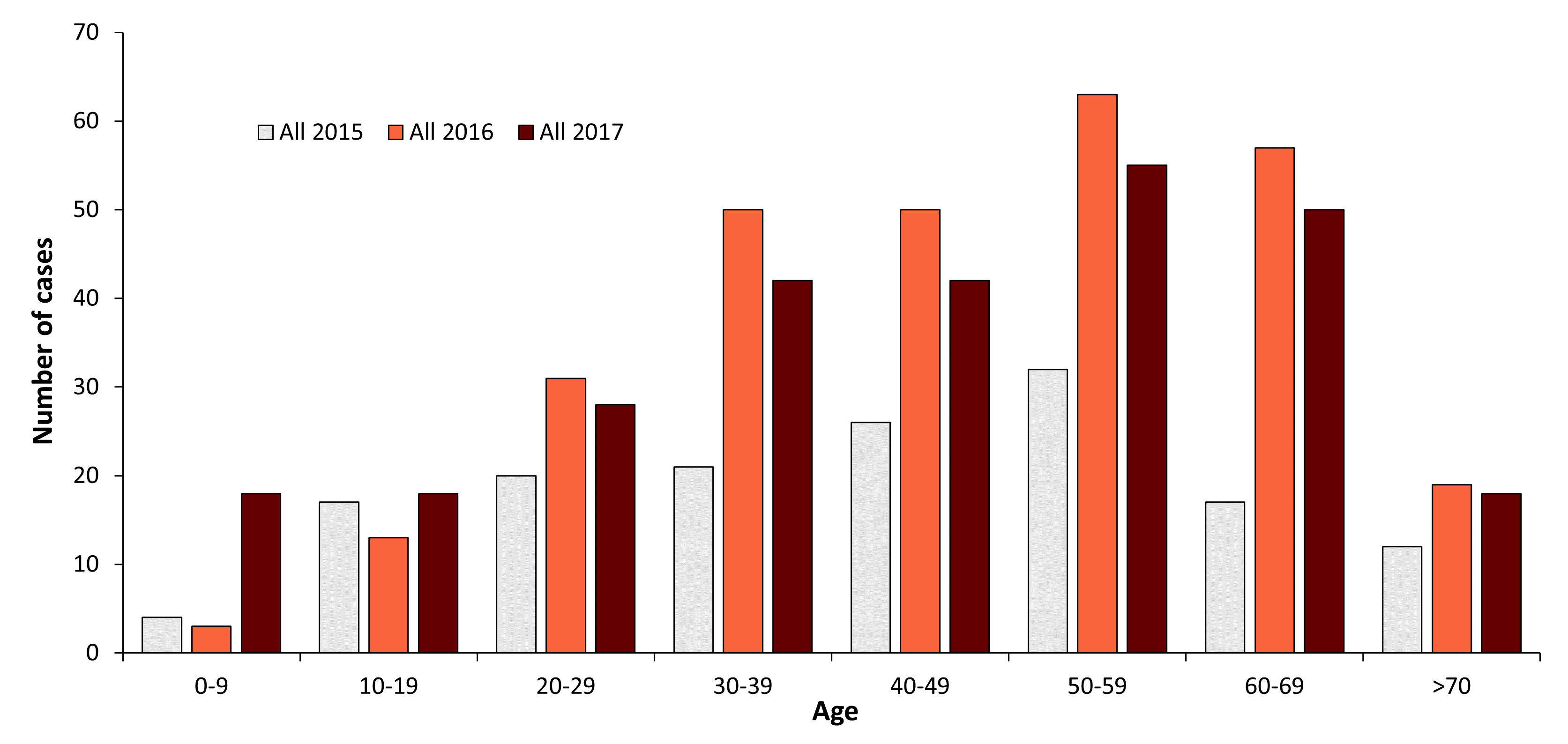
| Age group | Males | Females | All 2015 | All 2016 | All 2017 |
|---|---|---|---|---|---|
| 0-9 | - | - | 4 | 3 | 18 |
| 10-19 | - | - | 17 | 13 | 18 |
| 20-29 | - | - | 20 | 31 | 28 |
| 30-39 | - | - | 21 | 50 | 42 |
| 40-49 | - | - | 26 | 50 | 42 |
| 50-59 | - | - | 32 | 63 | 55 |
| 60-69 | - | - | 17 | 57 | 50 |
| >70 | - | - | 12 | 19 | 18 |
Contact:
kpancer@pzh.gov.pl
Citation:
Pancer K, Gut W. TBE in Poland. Chapter 12b. In: Dobler G, Erber W, Bröker M, Schmitt HJ, eds. The TBE Book. 6th ed. Singapore: Global Health Press; 2023. doi:10.33442/26613980_12b25-6
References
- Demiaszkiewicz W. Wiosenno-letnie kleszczowe zapalenie mózgu w Puszczy Białowieskiej. Pol Tyg Lek. 1952;7:799-801.
- Gut W, Prokopowicz D. Half century of the TBE in Poland. Przegl Epidemiol. 2002;56:129-35.
- Karbowiak G, Biernat B. The role of particular tick developmental stages in the circulation of tick-borne pathogens affecting humans in Central Europe. 2. Tick-borne encephalitis virus. Ann Parasitol. 2016;62:3-9.
- Katargina O, Russakova S, Geller J, et al. Detection and characterization of tick-borne encephalitis virus in Baltic countries and Eastern Poland. PLoS One. 2013;8:e61374.
- Kańtoch M. Studies on etiopathogenesis and immunity in viral infections. Zbl Bakt. 1994;281:365-79.
- Kowalewska A, Siwińska A, Gut W. Charakterystyka odpowiedzi immunologicznej dla wirusa kleszczowego zapalenia mózgu w nowych ogniskach zachorowań w 1993 i 1995 roku. Medycyna ogólna. 1997;3:283-9.
- Makówka A, Gut W, Stefanoff P. Detection of TBEV RNA in ticks as a tool for valuation of endemic area and sensitivity of TBE surveillance. Przegl Epidemiol. 2009;63:375-8.
- Prokopowicz D, Bobrowska E, Bobrowski M, Grzeszczuk A. Prevalence of antibodies among residents of North- Eastern Poland. Scand J Infect Dis. 1995;27:15-16.
- Stefanoff P, Siennicka J, Kaba J, Nowicki M, Ferenczi E, Gut W. Identification of new endemic tick-borne encephalitis foci in Poland. Int J Med Microbiol. 2008;281(S1):102-7.
- Biernat B, Cieniuch S, Stańczak J. Detection of TBEV RNA in Ixodes ricinus ticks in north-eastern Poland. Ann Agric Environ Med. 2014;21:689-92.
- Drelich A, Andreassen A, Vainio K, Kruszynski P, Wasik TJ. Prevalence of tick-borne encephalitis virus in a highly urbanized and low risk area in Southern Poland. Ticks Tick Borne Dis. 2014;5:663-7.
- Wojcik-Fatla A, Cisaka E, Zajac V, Zwolinski J, Dutkiewicz J. Prevalence of tick-borne encephalitis virus in Ixodes ricinus and Dermacentor reticulatus ticks collected from the Lublin region (eastern Poland). Ticks Tick-Borne Dis. 2011;2:16-19.
- Annual report: Infectious diseases and poisonings in Poland 2006-2014. NIZP- PZH, GIS 2007-2015. Available at: http://wwwold.pzh.gov.pl/oldpage/epimeld/index_p.html.
- Jeżyna C, Węglińska T, Nawrocka E, et al. Epidemia mleczna kleszczowego zapalenia mózgu w województwie olsztyńskim. Przegl Epidemiol. 1976;30:479-89.
- Tarnowska H, Matuszczyk I, Żabicka J, Gut W. The outbreak of a milk epidemic of encephalitis caused by tick-borne encephalitis virus in Kielce Province. Przegl Epidemiol. 1997;51:381-8.
- Grzeszczuk A, Sokolewicz-Bobrowska E, Prokopowicz D. Adverse reactions to Tick–borne encephalitis vaccine FSME-Immun. Infection. 1998;26:385-8.
- von Hedenström M, Heberle U, Theobald K. Vaccination against tick-borne encephalitis (TBE): influence of simultaneous application of TBE immunoglobulin on seroconversion and rate of adverse events. Vaccine. 1995;13:759-62.
- Annual report: Vaccinations in Poland NIZP- PZH, GIS 2016. Available at: http://wwwold.pzh.gov.pl/oldpage/epimeld/index_p.html.
- Decision 2012/506/EU Commission Implementing Decision of 8 August 2012 amending Decision 2002/253/ EC laying down case definitions for reporting communicable diseases to the Community network under Decision No 2119/98/EC of the European Parliament and of the Council. Official Journal of the European Union (EUR-Lex), September 27, 2012. Section 2.44 Tick-Borne Encephalitis (TBE virus); pp34-35. Available at: http://eur-lex.europa.eu/legal-content/EN/ TXT/PDF/?uri=OJ:L:2012:262:FULL&from=EN.
- Czarkowski MP, Cielebąk E, Staszewska-Jakubik E, Kondej B. Reports on cases of infectious diseases and poisonings in Poland (biweekly, quarterly, semi-annually, annually). Available at: http://wwwold.pzh.gov.pl/oldpage/epimeld/index_p.html
- Biernat B, Karbowiak G, Werszko J, Stanczak J. Prevalence of tick-borne encephalitis virus (TBEV) RNA in Dermacentor reticulatus ticks from natural and urban environment, Poland. Exp Appl Acarol. 2014; 64:543–551
- Biernat B, Karbowiak G, Stanczak J, Masny A, Werszko J, The first detection of the tick-borne encephalitis virus (TBEV) RNA in Dermacentor reticulatus ticks collected from the lowland European bison (Bison bonasus bonasus L.). Acta Parasitol. 2016;61:130-5.